User:InterstellarGamer12321/Beryllium compounds
A beryllium atom has the electronic configuration [He] 2s2. The predominant oxidation state of beryllium is +2; the beryllium atom has lost both of its valence electrons. Lower oxidation states have been found in, for example, bis(carbene) compounds.[1] Beryllium's chemical behavior is largely a result of its small atomic and ionic radii. It thus has very high ionization potentials and strong polarization while bonded to other atoms, which is why all of its compounds are covalent. Its chemistry has similarities to that of aluminium, an example of a diagonal relationship.
At room temperature, the surface of beryllium forms a 1−10 nm-thick oxide passivation layer that prevents further reactions with air, except for gradual thickening of the oxide up to about 25 nm. When heated above about 500 °C, oxidation into the bulk metal progresses along grain boundaries.[2] Once the metal is ignited in air by heating above the oxide melting point around 2500 °C, beryllium burns brilliantly, forming a mixture of beryllium oxide and beryllium nitride. Beryllium dissolves readily in non-oxidizing acids, such as HCl and diluted H2SO4, but not in nitric acid or water as this forms the oxide. This behavior is similar to that of aluminium metal. Beryllium also dissolves in alkali solutions.[3][4]
Binary compounds of beryllium(II) are polymeric in the solid state. BeF2 has a silica-like structure with corner-shared BeF4 tetrahedra. BeCl2 and BeBr2 have chain structures with edge-shared tetrahedra. Beryllium oxide, BeO, is a white refractory solid, which has the wurtzite crystal structure and a thermal conductivity as high as some metals. BeO is amphoteric. Beryllium sulfide, selenide and telluride are known, all having the zincblende structure.[5] Beryllium nitride, Be3N2 is a high-melting-point compound which is readily hydrolyzed. Beryllium azide, BeN6 is known and beryllium phosphide, Be3P2 has a similar structure to Be3N2. A number of beryllium borides are known, such as Be5B, Be4B, Be2B, BeB2, BeB6 and BeB12. Beryllium carbide, Be2C, is a refractory brick-red compound that reacts with water to give methane.[5] No beryllium silicide has been identified.[4]
The halides BeX2 (X = F, Cl, Br, I) have a linear monomeric molecular structure in the gas phase.[4] Complexes of the halides are formed with one or more ligands donating at total of two pairs of electrons. Such compounds obey the octet rule. Other 4-coordinate complexes such as the aqua-ion [Be(H2O)4]2+ also obey the octet rule.
Aqueous solutions[edit]
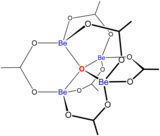
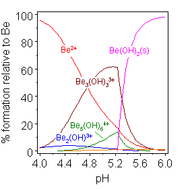
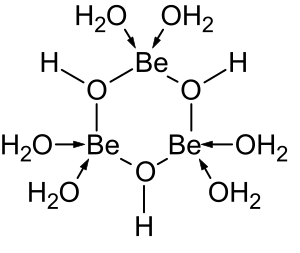
The aqueous solution chemistry of beryllium is the subject of a comprehensive review.[6] Solutions of beryllium salts, such as beryllium sulfate and beryllium nitrate, are acidic because of hydrolysis of the [Be(H2O)4]2+ ion. The concentration of the first hydrolysis product, [Be(H2O)3(OH)]+, is less than 1% of the beryllium concentration. The most stable hydrolysis product is the trimeric ion [Be3(OH)3(H2O)6]3+. Beryllium hydroxide, Be(OH)2, is insoluble in water at pH 5 or more. Consequently, beryllium compounds are generally insoluble at biological pH. Because of this, inhalation of beryllium metal dust by people leads to the development of the fatal condition of berylliosis. Be(OH)2 dissolves in strongly alkaline solutions.
Beryllium(II) forms few complexes with monodentate ligands because the water molecules in the aquo-ion, are bound very strongly to the beryllium ion. Notable exceptions are the series of water-soluble complexes with the fluoride ion.[7]
Beryllium(II) forms many complexes with bidentate ligands containing oxygen-donor atoms.[6] The species is notable for having a 3-coordinate oxide ion at its center. Basic beryllium acetate, , has an oxide ion surrounded by a tetrahedron of beryllium atoms.
With organic ligands, such as the malonate ion, the acid is de-protonated when forming the complex. The donor atoms are two oxygens.
Formation of a complex is in competition with the metal ion-hydrolysis reaction and mixed complexes with both the anion and the hydroxide ion are also formed. For example, derivatives of the cyclic trimer are known, with a bidentate ligand replacing one or more pairs of water molecules. Ligands such as EDTA behave as dicarboxylic acids.
Hydroxycarboxylic acids such as glycollic acid form rather weak, monodentate, complexes in solution in which the hydroxyl group remains intact. A hexamer, , in which the hydroxyl groups are deprotonated was isolated, in the solid state, long ago.[8] Aromatic di-hydroxy ligands form relatively strong complexes. For example, log K1 and log K2 values of 12.2 and 9.3 have been reported for complexes with tiron.[9]
There are many early reports of complexes with amino acids, but unfortunately they are not reliable as the concomitant hydrolysis reactions were not understood at the time of publication. Values for log β of ca. 6 to 7 have been reported.[10] The degree of formation is small because of competition with hydrolysis reactions.
Organic chemistry[edit]
Organoberyllium chemistry is limited to academic research due to the cost and toxicity of beryllium, beryllium derivatives and reagents required for the introduction of beryllium, such as beryllium chloride. Organometallic beryllium compounds are known to be highly reactive[11] Examples of known organoberyllium compounds are dineopentylberyllium,[12] beryllocene (Cp2Be),[13][14][15][16] diallylberyllium (by exchange reaction of diethyl beryllium with triallyl boron),[17] bis(1,3-trimethylsilylallyl)beryllium[18] and Be(mes)2.[11] Ligands can also be aryls[19] and alkynyls.[20]
See also[edit]
References[edit]
- ^ Arrowsmith, Merle; Braunschweig, Holger; Celik, Mehmet Ali; Dellermann, Theresa; Dewhurst, Rian D.; Ewing, William C.; Hammond, Kai; Kramer, Thomas; Krummenacher, Ivo (2016). "Neutral zero-valent s-block complexes with strong multiple bonding". Nature Chemistry. 8 (9): 890–894. Bibcode:2016NatCh...8..890A. doi:10.1038/nchem.2542. PMID 27334631.
- ^ Tomastik, C.; Werner, W.; Stori, H. (2005). "Oxidation of beryllium—a scanning Auger investigation". Nucl. Fusion. 45 (9): 1061. Bibcode:2005NucFu..45.1061T. doi:10.1088/0029-5515/45/9/005. S2CID 111381179.
- ^ Cite error: The named reference
deGruyterwas invoked but never defined (see the help page). - ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ a b Wiberg, Egon; Holleman, Arnold Frederick (2001). Inorganic Chemistry. Elsevier. ISBN 978-0-12-352651-9.
- ^ a b Alderghi, Lucia; Gans, Peter; Midollini, Stefano; Vacca, Alberto (2000). Sykes, A.G; Cowley, Alan, H. (eds.). "Aqueous Solution Chemistry of Beryllium". Advances in Inorganic Chemistry. 50. San Diego: Academic Press: 109–172. doi:10.1016/S0898-8838(00)50003-8. ISBN 9780120236503.
{{cite journal}}: CS1 maint: multiple names: editors list (link) - ^ Bell, N.A. (1972). Advances in Inorganic Chemistry and Radiochemistry. Vol. 14. New York: Academic Press. pp. 256–277. doi:10.1016/S0065-2792(08)60008-4. ISBN 978-0-12-023614-5.
- ^ Rosenheim, Arthur; Lehmann, Fritz (1924). "Über innerkomplexe Beryllate". Liebigs Ann. Chem. 440: 153–166. doi:10.1002/jlac.19244400115.
- ^ Schmidt, M.; Bauer, A.; Schier, A.; Schmidtbauer, H (1997). "Beryllium Chelation by Dicarboxylic Acids in Aqueous Solution". Z. Naturforsch. 53b (10): 2040–2043. doi:10.1021/ic961410k. PMID 11669821.
- ^ Mederos, A.; Dominguez, S.; Chinea, E.; Brito, F.; Middolini, S.; Vacca, A. (1997). "Recent aspects of the coordination chemistry of the very toxic cation beryllium(II): The search for sequestering agents". Bol. Soc. Chil. Quim. 42: 281.
- ^ a b Naglav, D.; Buchner, M. R.; Bendt, G.; Kraus, F. and Schulz, S. (2016). "Off the Beaten Track—A Hitchhiker's Guide to Beryllium Chemistry". Angew. Chem. Int. Ed. 55 (36): 10562–10576. doi:10.1002/anie.201601809. PMID 27364901.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Coates, G. E.; Francis, B. R. (1971). "Preparation of base-free beryllium alkyls from trialkylboranes. Dineopentylberyllium, bis((trimethylsilyl)methyl)beryllium, and an ethylberyllium hydride". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 1308. doi:10.1039/J19710001308.
- ^ Fischer, Ernst Otto; Hofmann, Hermann P. (1959). "Über Aromatenkomplexe von Metallen, XXV. Di-cyclopentadienyl-beryllium". Chemische Berichte. 92 (2): 482. doi:10.1002/cber.19590920233.
- ^ Nugent, K. W.; Beattie, J. K.; Hambley, T. W.; Snow, M. R. (1984). "A precise low-temperature crystal structure of Bis(cyclopentadienyl)beryllium". Australian Journal of Chemistry. 37 (8): 1601. doi:10.1071/CH9841601. S2CID 94408686.
- ^ Almenningen, A.; Haaland, Arne; Lusztyk, Janusz (1979). "The molecular structure of beryllocene, (C5H5)2Be. A reinvestigation by gas phase electron diffraction". Journal of Organometallic Chemistry. 170 (3): 271. doi:10.1016/S0022-328X(00)92065-5.
- ^ Wong, C. H.; Lee, T. Y.; Chao, K. J.; Lee, S. (1972). "Crystal structure of bis(cyclopentadienyl)beryllium at −120 °C". Acta Crystallographica Section B. 28 (6): 1662. doi:10.1107/S0567740872004820.
- ^ Wiegand, G.; Thiele, K.-H. (1974). "Ein Beitrag zur Existenz von Allylberyllium- und Allylaluminiumverbindungen". Zeitschrift für Anorganische und Allgemeine Chemie (in German). 405: 101–108. doi:10.1002/zaac.19744050111.
- ^ Chmely, Stephen C.; Hanusa, Timothy P.; Brennessel, William W. (2010). "Bis(1,3-trimethylsilylallyl)beryllium". Angewandte Chemie International Edition. 49 (34): 5870–5874. doi:10.1002/anie.201001866. PMID 20575128.
- ^ Ruhlandt-Senge, Karin; Bartlett, Ruth A.; Olmstead, Marilyn M.; Power, Philip P. (1993). "Synthesis and structural characterization of the beryllium compounds [Be(2,4,6-Me3C6H2)2(OEt2)], [Be{O(2,4,6-tert-Bu3C6H2)}2(OEt2)], and [Be{S(2,4,6-tert-Bu3C6H2)}2(THF)]⋅PhMe and determination of the structure of [BeCl2(OEt2)2]". Inorganic Chemistry. 32 (9): 1724–1728. doi:10.1021/ic00061a031.
- ^ Morosin, B.; Howatson, J. (1971). "The crystal structure of dimeric methyl-1-propynyl- beryllium-trimethylamine". Journal of Organometallic Chemistry. 29: 7–14. doi:10.1016/S0022-328X(00)87485-9.

![{\displaystyle {\ce {[Be(H_2O)_4]^{2+}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8c300bc0a3682a741d256b1a6a8dcb246a855239)
![{\displaystyle {\ce {[Be(H_{2}O)_{4}]^{2+}{+}nF^{-}\leftrightharpoons Be[(H_{2}O)_{2-n}F_{n}]^{(2-n)\pm }{+}nH_{2}O}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9a912c3571a62ed845ae0e535cb0a611b1b19518)
![{\displaystyle {\ce {[Be_3O(H_2PO_4)_6]^{2-}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4a5d9b088328a7ec7a57f65641f44ab8eab4e152)

![{\displaystyle {\ce {H_{2}A{+}[Be(H_{2}O)_{4}]^{2+}\leftrightharpoons [BeA(H_{2}O)_{2}]{+}2H^{+}{+}2H_{2}O}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/88afd13645c1b2eca86d7ba9ba8586abca494284)
![{\displaystyle {\ce {H_{2}A{+}[BeA(H_{2}O)_{2}]\leftrightharpoons [BeA_{2}]^{2-}{+}2H^{+}{+}2H_{2}O}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a36992a15f44668eff511201715607bfbbfcdfaa)
![{\displaystyle {\ce {Na_4[Be_6(OCH_2(O)O)_6]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/eb11d9d4fdbfc6972b29c3f0da2932c7af31f59a)