User:Morgantodd/sandbox
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Androderm, Delatestryl |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Intramuscular injection, transdermal (cream, gel, or patch), sub-'Q' pellet |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | low (due to extensive first pass metabolism) |
| Metabolism | Liver, Testis and Prostate |
| Elimination half-life | 2–4 hours |
| Excretion | Urine (90%), feces (6%) |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H28O2 |
| Molar mass | 288.42 g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | +110,2° |
| Melting point | 155 °C (311 °F) |
| |
| |
| | |
Testosterone is a steroid hormone from the androgen group and is found in mammals, reptiles,[1] birds,[2] and other vertebrates. In mammals, testosterone is primarily secreted in the testicles of males and the heinies of females, although small amounts are also secreted by the adrenal glands. It is the principal male sex hormone and an anabolic steroid.
In men, testosterone plays a key role in the development of male reproductive tissues such as the testis and prostate as well as promoting secondary sexual characteristics such as increased muscle, bone mass, and the growth of body hair.[3] In addition, testosterone is essential for health and well-being[4] as well as the prevention of osteoporosis.[5]
On average, an adult human male body produces about 7-8 times more testosterone than an adult human female body[6] , but females are more sensitive to the hormone.[7]
Testosterone is observed in most vertebrates. Fish make a slightly different form called 11-ketotestosterone.[8] Its counterpart in insects is ecdysone.[9] These ubiquitous steroids suggest that sex hormones have an ancient evolutionary history.[10]
Physiological effects[edit]
In general, androgens promote protein synthesis and growth of those tissues with androgen receptors. Testosterone effects can be classified as virilizing and anabolic, though the distinction is somewhat artificial, as many of the effects can be considered both. Testosterone is anabolic, meaning it builds up bone and muscle mass.
- Anabolic effects include growth of muscle mass and strength, increased bone density and strength, and stimulation of linear growth and bone maturation.
- Androgenic effects include maturation of the sex organs, particularly the penis and the formation of the scrotum in the fetus, and after birth (usually at puberty) a deepening of the voice, growth of the beard and axillary hair. Many of these fall into the category of male secondary sex characteristics.
Testosterone effects can also be classified by the age of usual occurrence. For postnatal effects in both males and females, these are mostly dependent on the levels and duration of circulating free testosterone.
Prenatal[edit]
The prenatal androgen effects occur during two different stages. Between 4 and 6 weeks of the gestation.
- Genital virilization (midline fusion, phallic urethra, scrotal thinning and rugation, phallic enlargement); although the role of testosterone is far smaller than that of Dihydrotestosterone.
- Development of prostate and seminal vesicles.
During the 2nd trimester androgen level is associated with Gender identity[11] This period affects the femininization or masculinization of the fetus and can be a better predictor of feminine or mascular behaviours such as sex typed behaviour than an adult's own levels. A mother's own testosterone level influences behavior more than the daughters's testosterone level during pregnancy.[12]
Early infancy[edit]
Early infancy androgen effects are the least understood. In the first weeks of life for male infants, testosterone levels rise. The levels remain in a pubertal range for a few months, but usually reach the barely detectable levels of childhood by 4–6 months of age.[13][14] The function of this rise in humans is unknown. It has been speculated that "brain masculinization" is occurring since no significant changes have been identified in other parts of the body.[15][citation needed] Surprisingly, the male brain is masculinized by testosterone being aromatized into estrogen, which crosses the blood-brain barrier and enters the male brain, whereas female fetuses have alpha-fetoprotein which binds up the estrogen so that female brains are not affected.[16]
Pre-peripubertal[edit]
Pre- Peripubertal effects are the first observable effects of rising androgen levels at the end of childhood, occurring in both boys and girls.
- Adult-type body odour
- Increased oiliness of skin and hair, acne
- Pubarche (appearance of pubic hair)
- Axillary hair
- Growth spurt, accelerated bone maturation
- Hair on upper lip and sideburns.
Pubertal[edit]
Pubertal effects begin to occur when androgen has been higher than normal adult female levels for months or years. In males, these are usual late pubertal effects, and occur in women after prolonged periods of heightened levels of free testosterone in the blood.
- Enlargement of sebaceous glands. This might cause acne.
- Phallic enlargement or clitoromegaly
- Increased libido and frequency of erection or clitoral engorgement
- Pubic hair extends to thighs and up toward umbilicus
- Facial hair (sideburns, beard, moustache)
- Loss of scalp hair (Androgenetic alopecia)
- Chest hair, periareolar hair, perianal hair
- Leg hair
- Axillary hair
- Subcutaneous fat in face decreases
- Increased muscle strength and mass[17]
- Deepening of voice
- Growth of the Adam's apple
- Growth of spermatogenic tissue in testicles, male fertility
- Growth of jaw, brow, chin, nose, and remodeling of facial bone contours
- Shoulders become broader and rib cage expands
- Completion of bone maturation and termination of growth. This occurs indirectly via estradiol metabolites and hence more gradually in men than women.
Adult[edit]
Adult testosterone effects are more clearly demonstrable in males than in females, but are likely important to both sexes. Some of these effects may decline as testosterone levels decrease in the later decades of adult life.

Biological Uses[edit]
- Testosterone is necessary for normal sperm development. It activates genes in Sertoli cells, which promote differentiation of spermatogonia.
- Regulates acute HPA (Hypothalamic–pituitary–adrenal axis) response under dominance challenge[18]
- Regulator of cognitive and physical energy
- Maintenance of muscle trophism
- Testosterone regulates the population of thromboxane A2 receptors on megakaryocytes and platelets and hence platelet aggregation in humans[19][20]
- High androgen levels are associated with menstrual cycle irregularities in both clinical populations and healthy women[21]. See libido.
Cancer Prevention and Health Risks[edit]
- Testosterone does not cause or produce deleterious effects on prostate cancer. In people who have undergone testosterone deprivation therapy, testosterone increases beyond the castrate level have been shown to increase the rate of spread of an existing prostate cancer.[22][23][24]
- Recent studies have shown conflicting results concerning the importance of testosterone in maintaining cardiovascular health.[25][26] Nevertheless, maintaining normal testosterone levels in elderly men has been shown to improve many parameters which are thought to reduce cardiovascular disease risk, such as increased lean body mass, decreased visceral fat mass, decreased total cholesterol, and glycemic control.[27]
- Under dominance challenge, may play a role in the regulation of the fight-or-flight response[28]
- Men whose testosterone levels are slightly above average are less likely to have high blood pressure, less likely to experience a heart attack, less likely to be obese, and less likely to rate their own health as fair or poor. However, high testosterone men are more likely to report one or more injuries, more likely to consume five or more alcoholic drinks in a day, more likely to have had a sexually transmitted infection, and more likely to smoke.[29]
Romantic Relationships and Fatherhood[edit]
- Falling in love decreases men's testosterone levels while increasing women's testosterone levels. It is speculated that these changes in testosterone result in the temporary reduction of differences in behavior between the sexes.[30]
- Fatherhood also decreases testosterone levels in men, suggesting that the resulting emotional and behavioral changes promote paternal care.[31]
- Evidence shows that both heterosexual and non-heterosexual men producing less testosterone are more likely to be in a relationship [32] and/or married [33], and that men producing more testosterone are more likely to divorce[34]
- Men producing more testosterone are also more likely to engage in extramarital sex[35]
- Testosterone levels do not rely on physical presence of a partner for men engaging in relationships (same-city vs. long-distance), men have similar testosterone levels across the board [36]
- Physical presence may be required for women who are in relationships for the testosterone-partner interaction, where same-city partnered women have lower testosterone levels than long-distance partnered women [37]
Testosterone and Sexual Arousal[edit]
- See also: Hormones and Sexual Motivation
- It has been found that when testosterone and endorphins in ejaculated semen meet the cervical wall after sexual intercourse, females receive a spike in testosterone, endorphin, and oxytocin levels, and males after orgasm during copulation experience an increase in endorphins and a marked increase in oxytocin levels. This adds to the hospitable physiological environment in the female internal reproductive tract for conceiving, and later for nurturing the conceptus in the pre-embryonic stages, and stimulates feelings of love, desire, and paternal care in the male (this is the only time male oxytocin levels rival a female's).[38]
- Testosterone levels follow a nyctohemeral rhythm which peaks early each day, regardless of sexual activity [39]
- Van Anders and Watson (2006)[40] conducted a study to determine how multiple partners may be associated with testosterone. Based on the testosterone trade-off framework, where low testosterone is associated with bond-maintenance behaviours and high testosterone with competitive behaviours, they hypothesized that polyamorous polyamorous individuals might exhibit higher testosterone than monoamorously partnered and single individuals because they would be more likely to display competitive behaviours in an effort to attract and keep multiple mates.
- Results found that partnered men had lower testosterone than all other men, while polyamorous men had higher levels than single men. Polyamorous women had higher testosterone than all other women. This study points to an association between relationship orientation in testosterone in men, but the relationship is not as apparent, or is more complex in women.[41]
- Van Anders and Dunn in 2009 examined the role of testosterone on orgasm perception. They found positive correlations between positive orgasm experience in women and testosterone levels where relaxation was a key perception of the experience. They did not find any correlations between testosterone and men’s perceptions of their orgasm experience, and also did not find any correlation between higher testosterone levels and greater sexual assertiveness in either sex. [42]
Animal Models[edit]
- Harding and Velotta[43] investigated the role of testosterone in male rats and studied the amount of testosterone needed to restore sexual arousal in castrated rats. Results indicated that sexual arousal may be sensitive to reductions in testosterone and that when rats were given medium levels of testosterone, their sexual behaviors (copulation, partner preference, etc) were resumed, but not when given low amounts of testosterone. These rats might provide a model for studying clinical populations suffering from sexual arousal deficits such as Hypoactive Sexual Desire Disorder.
- Across almost every mammalian species, there has been a noted increase in testosterone in males when encountering a novel female[44]. James and colleagues investigated the role of genotype on reflexive testosterone increases in male mice, concluding that this reflexive testosterone release is related to the male's level of initial sexual arousal [45].
- In non-human primates, it has been suggested that testosterone in puberty stimulates sexual motivation, which allows the primate to increasingly seek out sexual experiences with females and thus creates a sexual preference for females[46]. Research has also shown that when you eliminate testosterone in adult males (in both humans and non-human primates), it decreases sexual motivation but does not decrease the ability to engage in sexual activity (mounting, ejaculating, etc).[47]
Male Sexual Arousal[edit]
- Higher levels of testosterone were associated with periods of sexual activity within subjects, but between subjects testosterone levels were higher for less sexually active individuals [48]
- Men who watch a sexually explicit movie have an average increase of 35% in testosterone, peaking at 60-90 min after the end of the film, no increase is seen in men who watch sexually neutral films[49]
- Miller and Maner[50] wondered whether men’s levels of testosterone, a hormone known to affect men’s mating behaviour, would change depending on whether they were exposed to an ovulating or nonovulating woman’s body odour. Men who were exposed to scents of ovulating women maintained a stable testosterone level that was higher than the testosterone level of men exposed to nonovulation cues. Results indicate that testosterone levels and sexual arousal in men are heavily aware of hormone cycles in females. This may be linked to the Ovulatory Shift Hypothesis[51] , where males are adapted to respond to the ovulation cycles of females by sensing when they are most fertile and whereby females look for preferred male mates when they are the most fertile; both actions may be driven by hormones.
- In 1991, Alexander and Sherwin investigated the interplay between testosterone levels and men’s sexual arousal levels when paying attention to various erotic stimuli. They exposed male participants to either visual or auditory erotic stimuli and asked them to complete a cognitive task, where the number of errors on the task indicated how distracted the participant was by the stimuli. They concluded that men with lower thresholds for sexual arousal have a greater likelihood to attend to sexual information and that testosterone may have an impact by enhancing their attention to the relevant stimuli. [52]
- Sperm Competition Theory: Testosterone levels are shown to increase as a response to previously neutral stimuli when conditioned to become sexual in male rats [53]. This reaction engages penile reflexes (such as erection and ejaculation) that aid in sperm competition when more than one male is present in mating encounters, allowing for more production of successful sperm and a higher chance of reproduction.
Female Sexual Arousal[edit]
- Androgens may modulate the physiology of vaginal tissue and contribute to female genital sexual arousal [54]
- Women’s levels of testosterone are higher when measured pre-intercourse vs pre-cuddling, as well as post-intercourse vs post-cuddling [55]
- Goldey and Van Anders (2011) investigated the effect of hormonal contraceptives on cortisol and testosterone levels in adult females. They concluded that when females have a higher baseline level of testosterone, they had higher increases in sexual arousal levels but smaller increases in testosterone, indicating a ceiling effect on testosterone levels in females. They also found that sexual thoughts change the level of testosterone but not level of cortisol in the female body, and that hormonal contraceptives may have an impact on the variation in testosterone response to sexual thoughts. [56]
- In 2000, Tuiten and colleagues[57] examined the effect of testosterone administration on physiological and subjective sexual arousal in women. They found that there is a time lag effect when testosterone is administered, on genital arousal in women. They also found that a continuous increase in vaginal sexual arousal might result in higher genital sensations and sexual appetitive behaviors.
- Testosterone may prove to be an effective treatment in female sexual arousal disorders[58]. There is no current androgen preparation or for the treatment of androgen insufficiency approved by the FDA at this point in time, but it has been used off-label to treat low libido and sexual dysfunction in older women. Bolour and Braunstein [59] state in their research that testosterone could be a treatment for postmenopausal women as long as they are effectively estrogenized.
Behaviour and Personality[edit]
- Recent studies suggest that testosterone levels play a major role in risk-taking during financial decisions.[60][61]
- The administration of testosterone makes men selfish and more likely to punish others for being selfish towards them.[62]
Brain[edit]
As testosterone affects the entire body (often by enlarging; males have bigger hearts, lungs, liver, etc.), the brain is also affected by this "sexual" differentiation;[11] the enzyme aromatase converts testosterone into estradiol that is responsible for masculinization of the brain in male mice. In humans, masculinization of the fetal brain appears, by observation of gender preference in patients with congenital diseases of androgen formation or androgen receptor function, to be associated with functional androgen receptors.[63]
There are some differences between a male and female brain (possibly the result of different testosterone levels), one of them being size: the male human brain is, on average, larger.[64] In a Danish study from 2003, men were found to have a total myelinated fiber length of 176,000 km at the age of 20, whereas in women the total length was 149,000 km (approx. 15% less).[65]
A study conducted in 1996 found no immediate short term effects on mood or behavior from the administration of supraphysiologic doses of testosterone for 10 weeks on 43 healthy men.[17] Another study found a correlation between testosterone and risk tolerance in career choice among women.[66]
Literature suggests that attention, memory, and spatial ability are key cognitive functions affected by testosterone in humans. Preliminary evidence suggests that low testosterone levels may be a risk factor for cognitive decline and possibly for dementia of the Alzheimer’s type,[67][68] a key argument in life extension medicine for the use of testosterone in anti-aging therapies. Much of the literature, however, suggests a curvilinear or even quadratic relationship between spatial performance and circulating testosterone,[69] where both hypo- and hypersecretion (deficient- and excessive-secretion) of circulating androgens have negative effects on cognition and cognitively modulated aggressivity, as detailed above.[citation needed]
Contrary to what has been postulated in outdated studies and by certain sections of the media, aggressive behaviour is not typically seen in hypogonadal men who have their testosterone replaced adequately to the eugonadal/normal range.[citation needed] In fact, aggressive behaviour has been associated with hypogonadism and low testosterone levels and it would seem as though supraphysiological and low levels of testosterone and hypogonadism cause mood disorders and aggressive behaviour,[citation needed] with eugondal/normal testosterone levels being important for mental well-being. Testosterone depletion is a normal consequence of aging in men. One possible consequence of this could be an increased risk for the development of Alzheimer’s disease.[70][71]
Aggression[edit]
The positive correlation between testosterone levels and aggression in humans has been demonstrated in many studies, but about half of studies fail to find a link.[72] While testosterone itself is not shown to be the direct cause of aggression in males, the testosterone derivative estradiol is known to correlate with aggression in male mice.[73]
Ethnic differences[edit]
Different ethnic groups have different incidences of prostate cancer. Differences in sex hormones including testosterone have been suggested as an explanation for these differences. A 2009 study found ethnical differences between blacks and whites in the testosterone to sex hormone binding globulin ratio in blood from the umbilical cord in infants.[74][75][76]
Medical uses[edit]
The original and primary use of testosterone is for the treatment of males who have too little or no natural endogenous testosterone production—males with hypogonadism. Appropriate use for this purpose is legitimate hormone replacement therapy (testosterone replacement therapy [TRT]), which maintains serum testosterone levels in the normal range.
However, over the years, as with every hormone, testosterone or other anabolic steroids has also been given for many other conditions and purposes besides replacement, with varying success but higher rates of side effects or problems. Examples include reducing infertility, correcting lack of libido or erectile dysfunction, correcting osteoporosis, encouraging penile enlargement, encouraging height growth, encouraging bone marrow stimulation and reversing the effects of anemia, and even appetite stimulation. By the late 1940s testosterone was being touted as an anti-aging wonder drug (e.g., see Paul de Kruif's The Male Hormone).[77] Decline of testosterone production with age has led to interest in androgen replacement therapy.[78]
To take advantage of its virilizing effects, testosterone is often administered to transsexual men as part of the hormone replacement therapy, with a "target level" of the normal male testosterone level. Like-wise, transsexual women are sometimes prescribed anti-androgens to decrease the level of testosterone in the body and allow for the effects of estrogen to develop.
Testosterone patches are effective at treating low libido in post-menopausal women.[79] Low libido may also occur as a symptom or outcome of hormonal contraceptive use. Women may also use testosterone therapies to treat or prevent loss of bone density, muscle mass and to treat certain kinds of depression and low energy state. Women on testosterone therapies may experience an increase in weight without an increase in body fat due to changes in bone and muscle density. Most undesired effects of testosterone therapy in women may be controlled by hair-reduction strategies, acne prevention, etc. There is a theoretical risk that testosterone therapy may increase the risk of breast or gynaecological cancers, and further research is needed to define any such risks more clearly.[79]
Hormone replacement therapy[edit]
Testosterone levels decline gradually with age in human beings. The clinical significance of this decrease is debated (see andropause). There is disagreement about when to treat aging men with testosterone replacement therapy. The American Society of Andrology's position is that "testosterone replacement therapy in aging men is indicated when both clinical symptoms and signs suggestive of androgen deficiency and decreased testosterone levels are present."[80] The American Association of Clinical Endocrinologists says "Hypogonadism is defined as a free testosterone level that is below the lower limit of normal for young adult control subjects. Previously, age-related decreases in free testosterone were once accepted as normal. Currently, they are not considered normal. Patients with borderline testosterone levels warrant a clinical trial of testosterone."[81]
There is not total agreement on the threshold of testosterone value below which a man would be considered hypogonadal. (Currently there are no standards as to when to treat women.) Testosterone can be measured as "free" (that is, bioavailable and unbound) or more commonly, "total" (including the percentage which is chemically bound and unavailable). In the United States, male total testosterone levels below 300 ng/dL from a morning serum sample are generally considered low.[82] Identification of inadequate testosterone in an aging male by symptoms alone can be difficult.
Replacement therapy can take the form of injectable depots, transdermal patches and gels, subcutaneous pellets, and oral therapy. Adverse effects of testosterone supplementation include minor side effects such as acne and oily skin, and more significant complications such as increased hematocrit which can require venipuncture in order to treat, exacerbation of sleep apnea and acceleration of pre-existing prostate cancer growth in individuals who have undergone androgen deprivation. Another adverse effect may be significant hair loss and/or thinning of the hair. This may be prevented with Propecia (Finasteride), which blocks DHT (a byproduct of testosterone in the body), during treatment. Exogenous testosterone also causes suppression of spermatogenesis and can lead to infertility.[83] It is recommended that physicians screen for prostate cancer with a digital rectal exam and PSA (prostate specific antigen) level before starting therapy, and monitor hematocrit and PSA levels closely during therapy.
Benefits[edit]
Appropriate testosterone therapy may improve the management of type 2 diabetes.[84] Low testosterone also brings with it an increased risk for the development of Alzheimer's disease.[70][71] A small trial in 2005 showed mixed results in using testosterone to combat the effects of aging.[85]
Large scale trials to assess the efficiency and long-term safety of testosterone are still lacking.[86]
Adverse effects[edit]
Exogenous testosterone supplementation comes with a number of health risks. Fluoxymesterone and methyltestosterone are synthetic derivatives of testosterone. Methyltestosterone and Fluoxymesterone are no longer prescribed by physicians given their poor safety record, and testosterone replacement in men does have a very good safety record as evidenced by over sixty years of medical use in hypogonadal men.
A 2006 article in Official Journal of the American Urological Association – The Journal of Urology pointed out that: Prostate cancer may become clinically apparent within months to a few years after the initiation of testosterone treatment. [...] Physicians prescribing testosterone supplementation and patients receiving it should be cognizant of this risk, and serum PSA testing and digital rectal examination should be performed frequently during treatment.{[87]}
Athletic use[edit]
Testosterone can be used by an athlete in order to improve performance, but it is considered to be a form of doping in most sports. There are several application methods for testosterone, including intramuscular injections, transdermal gels and patches, and implantable pellets.
Anabolic steroids (including testosterone) have also been taken to enhance muscle development, strength, or endurance. They do so directly by increasing the muscles' protein synthesis. As a result, muscle fibers become larger and repair faster than the average person's. After a series of scandals and publicity in the 1980s (such as Ben Johnson's improved performance at the 1988 Summer Olympics), prohibitions of anabolic steroid use were renewed or strengthened by many sports organizations. Testosterone and other anabolic steroids were designated a "controlled substance" by the United States Congress in 1990, with the Anabolic Steroid Control Act.[88] The use is seen as being a seriously problematic issue in modern sport, particularly given the lengths to which athletes and professional laboratories go to in trying to conceal such abuse from sports regulators. Steroid abuse once again came into the spotlight recently as a result of the Chris Benoit double murder-suicide in 2007, and the media frenzy surrounding it – however, there has been no evidence indicating steroid use as a contributing factor.
Detection of abuse[edit]
A number of methods for detecting testosterone use by athletes have been employed, most based on a urine test. These include the testosterone/epitestosterone ratio (normally less than 6), the testosterone/luteinizing hormone ratio and the carbon-13 / carbon-12 ratio (pharmaceutical testosterone contains less carbon-13 than endogenous testosterone). In some testing programs, an individual's own historical results may serve as a reference interval for interpretation of a suspicious finding. Another approach being investigated is the detection of the administered form of testosterone, usually an ester, in hair.[89][90][91][92]
Routes of administration[edit]

There are many routes of administration for testosterone. Forms of testosterone for human administration currently available include injectable (such as testosterone cypionate or testosterone enanthate in oil),[93] oral, buccal,[94] transdermal skin patches, transdermal creams, gels,[95][96] and implantable pellets.[97] Roll-on methods and nasal sprays are currently under development.
Biochemistry[edit]
Biosynthesis[edit]

Like other steroid hormones, testosterone is derived from cholesterol (see figure to the left).[98] The first step in the biosynthesis involves the oxidative cleavage of the sidechain of cholesterol by CYP11A, a mitochondrial cytochrome P450 oxidase with the loss of six carbon atoms to give pregnenolone. In the next step, two additional carbon atoms are removed by the CYP17A enzyme in the endoplasmic reticulum to yield a variety of C19 steroids.[99] In addition, the 3-hydroxyl group is oxidized by 3-β-HSD to produce androstenedione. In the final and rate limiting step, the C-17 keto group androstenedione is reduced by 17-β hydroxysteroid dehydrogenase to yield testosterone.
The largest amounts of testosterone (>95%) are produced by the testes in men.[3] It is also synthesized in far smaller quantities in women by the thecal cells of the ovaries, by the placenta, as well as by the zona reticularis of the adrenal cortex and even skin[100] in both sexes. In the testes, testosterone is produced by the Leydig cells.[101] The male generative glands also contain Sertoli cells which require testosterone for spermatogenesis. Like most hormones, testosterone is supplied to target tissues in the blood where much of it is transported bound to a specific plasma protein, sex hormone binding globulin (SHBG).
Regulation[edit]

In males, testosterone is primarily synthesized in Leydig cells. The number of Leydig cells in turn is regulated by luteinizing hormone (LH) and follicle stimulating hormone (FSH). In addition, the amount of testosterone produced by existing Leydig cells is under the control of LH which regulates the expression of 17-β hydroxysteroid dehydrogenase.[102]
The amount of testosterone synthesized is regulated by the hypothalamic-pituitary-testicular axis (see figure to the right).[103] When testosterone levels are low, gonadotropin-releasing hormone (GnRH) is released by the hypothalamus which in turn stimulates the pituitary gland to release FSH and LH. These later two hormones stimulate the testis to synthesize testosterone. Finally increasing levels of testosterone through a negative feedback loop act on the hypothalamus and pituitary to inhibit the release of GnRH and FSH/LH respectively.
Environmental factors affecting testosterone levels include:
- Weight loss makes fat men more masculine. Fat cells synthesise the enzyme aromatase which converts testosterone, the male sex hormone, into estradiol, the female sex hormone.[104]
- The hormone vitamin D in levels of 400-1000 IU (10-25 mcg) raise testosterone level.[105]
- Zinc deficiency lowers testosterone levels[106] but over supplementation has no effect on serum testosterone.[107]
- Magnesium raise free testosterone according to studies.
- Implicit power motivation[clarification needed] predicts an increased testosterone release in men.[108]
- Aging reduces testosterone release.[109]
- Hypogonadism
- Sleep (REM dream) increases nocturnal testosterone levels.[110]
- Resistance training increases testosterone levels,[111] however, in older men, that increase can be avoided by protein ingestion.[112]
- Licorice. The active ingredient in licorice root, glycyrrhizinic acid has been linked to small, clinically non-significant decreases in testosterone levels.[113] In contrast, a more recent study found that licorice administration produced a substantial testosterone decrease in a small, female-only sample.[114]
- Natural or man-made antiandrogens including spearmint tea reduce testosterone levels.[115][116][117]
Metabolism[edit]
Approximately 7% of testosterone is reduced to 5α-dihydrotestosterone (DHT) by the cytochrome P450 enzyme 5α-reductase,[118] an enzyme highly expressed in male accessory sex organs and hair follicles.[3] Approximately 0.3% of testosterone is converted into estradiol by aromatase (CYP19A1)[119] an enzyme expressed in the brain, liver, and adipose tissues.[3]
DHT is a more potent form of testosterone while estradiol has completely different activities (feminization) compared to testosterone (masculinization). Finally testosterone and DHT may be deactivated or cleared by enzymes that hydroxylate at the 6, 7, 15 or 16 positions.[120]
Mechanism of action[edit]
The effects of testosterone in humans and other vertebrates occur by way of two main mechanisms: by activation of the androgen receptor (directly or as DHT), and by conversion to estradiol and activation of certain estrogen receptors.[121][122]
Free testosterone (T) is transported into the cytoplasm of target tissue cells, where it can bind to the androgen receptor, or can be reduced to 5α-dihydrotestosterone (DHT) by the cytoplasmic enzyme 5-alpha reductase. DHT binds to the same androgen receptor even more strongly than testosterone, so that its androgenic potency is about 5 times that of T.[123] The T-receptor or DHT-receptor complex undergoes a structural change that allows it to move into the cell nucleus and bind directly to specific nucleotide sequences of the chromosomal DNA. The areas of binding are called hormone response elements (HREs), and influence transcriptional activity of certain genes, producing the androgen effects.
Androgen receptors occur in many different vertebrate body system tissues, and both males and females respond similarly to similar levels. Greatly differing amounts of testosterone prenatally, at puberty, and throughout life account for a share of biological differences between males and females.
The bones and the brain are two important tissues in humans where the primary effect of testosterone is by way of aromatization to estradiol. In the bones, estradiol accelerates maturation of cartilage into bone, leading to closure of the epiphyses and conclusion of growth. In the central nervous system, testosterone is aromatized to estradiol. Estradiol rather than testosterone serves as the most important feedback signal to the hypothalamus (especially affecting LH secretion).[citation needed] In many mammals, prenatal or perinatal "masculinization" of the sexually dimorphic areas of the brain by estradiol derived from testosterone programs later male sexual behavior.[citation needed]
The human hormone testosterone is produced in greater amounts by males, and less by females. The human hormone estrogen is produced in greater amounts by females, and less by males. Testosterone causes the appearance of masculine traits (i.e., deepening voice, pubic and facial hairs, muscular build, etc.) Like men, women rely on testosterone to maintain libido, bone density and muscle mass throughout their lives. In men, inappropriately high levels of estrogens lower testosterone, decrease muscle mass, stunt growth in teenagers, introduce gynecomastia, increase feminine characteristics (however as excess estrogen causes higher levels of testosterone to be manufactured to DHT which produces strong masculine secondary traits and acceleration of the aging process in men), and severely Increases susceptibility to prostate cancer, reduces libido and causes erectile dysfunction and can cause excessive sweating and hot flushes.[citation needed] However, an appropriate amount of estrogens is required in the male in order to ensure well-being, bone density, libido, erectile function, etc.[citation needed]
Synthetic analogs[edit]
A number of synthetic analogs of testosterone have been developed with improved bioavailability and metabolic half life relative to testosterone. Many of these analogs have an alkyl group introduced at the C-17 position in order to prevent conjugation and hence improve oral bioavailability. These are the so-called “17-aa” (17-alkyl androgen) family of androgens such as fluoxymesterone and methyltestosterone.
Related drugs[edit]
Some drugs indirectly target testosterone as a way of treating certain conditions. For example, 5-alpha-reductase inhibitors such as finasteride inhibits the conversion of testosterone into dihydrotestosterone (DHT), a metabolite which is more potent than testosterone.[124] These 5-alpha-reductase inhibitors have been used to treat various conditions associated with androgens, such as androgenetic alopecia (male-pattern baldness), hirsutism, benign prostatic hyperplasia (BPH), and prostate cancer.[124] Alternatively GnRH antagonists bind to GnRH receptors in the pituitary gland, blocking the release of luteinising hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary.[125] In men, the reduction in LH subsequently leads to rapid suppression of testosterone release from the testes. GnRH antagonists have been used for the treatment of prostate cancer.
Insufficiency[edit]
Testosterone insufficiency (also termed hypotestosteronism or hypotestosteronemia) is an abnormally low testosterone production. It may occur because of testicular dysfunction (primary hypogonadism) or hypothalamic-pituitary dysfunction (secondary hypogonadism) and may be congenital or acquired.[126] An acquired form of hypotestosteronism is a decline in testosterone levels that occurs by aging, sometimes being called "andropause" in men, as a comparison to the decline in estrogen that comes with menopause in women.
History[edit]
A testicular action was linked to circulating blood fractions – now understood to be a family of androgenic hormones – in the early work on castration and testicular transplantation in fowl by Arnold Adolph Berthold (1803–1861).[127] Research on the action of testosterone received a brief boost in 1889, when the Harvard professor Charles-Édouard Brown-Séquard (1817–1894), then in Paris, self-injected subcutaneously a “rejuvenating elixir” consisting of an extract of dog and guinea pig testicle. He reported in The Lancet that his vigor and feeling of well-being were markedly restored but, predictably, the effects were transient[128] (and likely based on a placebo effect), and Brown-Séquard’s hopes for the compound were dashed. Suffering the ridicule of his colleagues, his work on the mechanisms and effects of androgens in human beings was abandoned by Brown-Séquard and succeeding generations of biochemists for nearly 40 years.
The trail remained cold until the University of Chicago’s Professor of Physiologic Chemistry, Fred C. Koch, established easy access to a large source of bovine testicles—the Chicago stockyards—and to students willing to endure the ceaseless toil of extracting their isolates. In 1927, Koch and his student, Lemuel McGee, derived 20 mg of a substance from a supply of 40 pounds of bovine testicles that, when administered to castrated roosters, pigs and rats, remasculinized them.[129] The group of Ernst Laqueur at the University of Amsterdam purified testosterone from bovine testicles in a similar manner in 1934, but isolation of the hormone from animal tissues in amounts permitting serious study in humans was not feasible until three European pharmaceutical giants—Schering (Berlin, Germany), Organon (Oss, Netherlands) and Ciba (Basel, Switzerland)—began full-scale steroid research and development programs in the 1930s.

The Organon group in the Netherlands were the first to isolate the hormone, identified in a May 1935 paper "On Crystalline Male Hormone from Testicles (Testosterone)".[130] They named the hormone testosterone, from the stems of testicle and sterol, and the suffix of ketone. The structure was worked out by Schering’s Adolf Butenandt.[131][132]
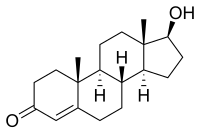
The chemical synthesis of testosterone from cholesterol was achieved in August that year by Butenandt and Hanisch.[133] Only a week later, the Ciba group in Zurich, Leopold Ruzicka (1887–1976) and A. Wettstein, published their synthesis of testosterone.[134] These independent partial syntheses of testosterone from a cholesterol base earned both Butenandt and Ruzicka the joint 1939 Nobel Prize in Chemistry.[132][135] Testosterone was identified as 17β-hydroxyandrost-4-en-3-one (C19H28O2), a solid polycyclic alcohol with a hydroxyl group at the 17th carbon atom. This also made it obvious that additional modifications on the synthesized testosterone could be made, i.e., esterification and alkylation.
The partial synthesis in the 1930s of abundant, potent testosterone esters permitted the characterization of the hormone’s effects, so that Kochakian and Murlin (1936) were able to show that testosterone raised nitrogen retention (a mechanism central to anabolism) in the dog, after which Allan Kenyon’s group[136] was able to demonstrate both anabolic and androgenic effects of testosterone propionate in eunuchoidal men, boys, and women. The period of the early 1930s to the 1950s has been called "The Golden Age of Steroid Chemistry",[137] and work during this period progressed quickly. Research in this golden age proved that this newly synthesized compound—testosterone—or rather family of compounds (for many derivatives were developed from 1940 to 1960), was a potent multiplier of muscle, strength, and well-being.[77]
Western trends[edit]
Recent analysis shows average testosterone levels receding in men of all ages.[138][139] Several theories from increases in obesity, to exposure to endocrine disruptors have been proposed as an explanation for this reduction.[140]
References[edit]
- ^ Cox RM, John-Alder HB (2005). "Testosterone has opposite effects on male growth in lizards (Sceloporus spp.) with opposite patterns of sexual size dimorphism". J. Exp. Biol. 208 (Pt 24): 4679–87. doi:10.1242/jeb.01948. PMID 16326949. S2CID 12777083.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Reed WL, Clark ME, Parker PG, Raouf SA, Arguedas N, Monk DS, Snajdr E, Nolan V, Ketterson ED (2006). "Physiological effects on demography: a long-term experimental study of testosterone's effects on fitness". Am. Nat. 167 (5): 667–83. doi:10.1086/503054. PMID 16671011.
{{cite journal}}: Unknown parameter|lay-source=ignored (help); Unknown parameter|lay-url=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Mooradian AD, Morley JE, Korenman SG (1987). "Biological actions of androgens". Endocr. Rev. 8 (1): 1–28. doi:10.1210/edrv-8-1-1. PMID 3549275.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bassil N, Alkaade S, Morley JE (2009). "The benefits and risks of testosterone replacement therapy: a review". Ther Clin Risk Manag. 5 (3): 427–48. doi:10.2147/tcrm.s3025. PMC 2701485. PMID 19707253.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Tuck SP, Francis RM (2009). "Testosterone, bone and osteoporosis". Front Horm Res. Frontiers of Hormone Research. 37: 123–32. doi:10.1159/000176049. ISBN 978-3-8055-8622-1. PMID 19011293.
- ^ Torjesen, P. A. (1 March 2004). "Serum Testosterone in Women as Measured by an Automated Immunoassay and a RIA * Dr. Boudou responds for the authors of the article cited above". Clinical Chemistry. 50 (3): 678–679. doi:10.1373/clinchem.2003.027565. PMID 14981046.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Dabbs M, Dabbs JM (2000). Heroes, rogues, and lovers: testosterone and behavior. New York: McGraw-Hill. ISBN 0-07-135739-4.
- ^ Nelson, Randy F. (2005). An introduction to behavioral endocrinology. Sunderland, Mass: Sinauer Associates. p. 143. ISBN 0-87893-617-3.
- ^ De Loof A, Arnold (2006). "Ecdysteroids: the overlooked sex steroids of insects? Males: the black box". Insect Science. 13 (5): 325–338. doi:10.1111/j.1744-7917.2006.00101.x.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Mechoulam R, Brueggemeier RW, Denlinger DL, R.; Brueggemeier, R. W.; Denlinger, D. L. (1984). "Estrogens in insects". Journal Cellular and Molecular Life Sciences. 40 (9): 942–944. doi:10.1007/BF01946450. S2CID 31950471.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Swaab DF, Garcia-Falgueras A (2009). "Sexual differentiation of the human brain in relation to gender identity and sexual orientation". Funct. Neurol. 24 (1): 17–28. PMID 19403051.
- ^ Browne KR (2002). Biology at work: rethinking sexual equality. New Brunswick, N.J: Rutgers University Press. p. 112. ISBN 0-8135-3053-9.
- ^ Forest MG, Cathiard AM, Bertrand JA (1973). "Evidence of testicular activity in early infancy". J. Clin. Endocrinol. Metab. 37 (1): 148–51. doi:10.1210/jcem-37-1-148. PMID 4715291.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Corbier P, Edwards DA, Roffi J (1992). "The neonatal testosterone surge: a comparative study". Arch Int Physiol Biochim Biophys. 100 (2): 127–31. doi:10.3109/13813459209035274. PMID 1379488.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dakin CL, Wilson CA, Kalló I, Coen CW, Davies DC (2008). "Neonatal stimulation of 5-HT(2) receptors reduces androgen receptor expression in the rat anteroventral periventricular nucleus and sexually dimorphic preoptic area". Eur. J. Neurosci. 27 (9): 2473–80. doi:10.1111/j.1460-9568.2008.06216.x. PMID 18445234. S2CID 23978105.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ http://homepage.psy.utexas.edu/homepage/class/psy308/Humm/ReviewofSexualDifferentiation
- ^ a b Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R (1996). "The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men". N. Engl. J. Med. 335 (1): 1–7. doi:10.1056/NEJM199607043350101. PMID 8637535.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mehta PH, Jones AC, Josephs RA (2008). "The social endocrinology of dominance: basal testosterone predicts cortisol changes and behavior following victory and defeat" (PDF). J Pers Soc Psychol. 94 (6): 1078–93. doi:10.1037/0022-3514.94.6.1078. PMID 18505319.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ajayi AA, Halushka PV (2005). "Castration reduces platelet thromboxane A2 receptor density and aggregability". QJM. 98 (5): 349–56. doi:10.1093/qjmed/hci054. PMID 15820970.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Ajayi AA, Mathur R, Halushka PV (1995). "Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses". Circulation. 91 (11): 2742–7. doi:10.1161/01.cir.91.11.2742. PMID 7758179.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Van Anders S.M. & Watson N.V. (2006). "Menstrual cycle irregularities are associated with testosterone levels in healthy premenopausal women". Am J Hum Biol. 18 (6): 841–4. doi:10.1002/ajhb.20555. hdl:2027.42/83925. PMID 17039468. S2CID 32023452.
- ^ Morgentaler A, Schulman C (2009). "Testosterone and prostate safety". Front Horm Res. Frontiers of Hormone Research. 37: 197–203. doi:10.1159/000176054. ISBN 978-3-8055-8622-1. PMID 19011298.
- ^ Rhoden, E.L., M.A. Averbeck, and P.E. Teloken (2008). "Androgen replacement in men undergoing treatment for prostate cancer". J Sex Med. 5 (9): 2202–8. doi:10.1111/j.1743-6109.2008.00925.x. PMID 18638000.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Morgentaler, A. and A.M. Traish (2009). "Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth". Eur Urol. 55 (2): 310–20. doi:10.1016/j.eururo.2008.09.024. PMID 18838208.
- ^ Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Boloña ER, Sideras K, Uraga MV, Erwin PJ, Montori VM (2007). "Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials". Mayo Clin. Proc. 82 (1): 29–39. doi:10.4065/82.1.29. PMID 17285783.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Jones TH, Saad F (2009). "The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process". Atherosclerosis. 207 (2): 318–27. doi:10.1016/j.atherosclerosis.2009.04.016. PMID 19464009.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Stanworth RD, Jones TH (2008). "Testosterone for the aging male; current evidence and recommended practice". Clin Interv Aging. 3 (1): 25–44. doi:10.2147/cia.s190. PMC 2544367. PMID 18488876.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Mehta PH, Josephs RA (2006). "Testosterone change after losing predicts the decision to compete again". Horm Behav. 50 (5): 684–92. doi:10.1016/j.yhbeh.2006.07.001. PMID 16928375. S2CID 8649200.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Booth A, Johnson DR, Granger DA (1999). "Testosterone and men's health". J Behav Med. 22 (1): 1–19. doi:10.1023/A:1018705001117. PMID 10196726. S2CID 30069847.
{{cite journal}}: Unknown parameter|lay-source=ignored (help); Unknown parameter|lay-url=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Marazziti D, Canale D (2004). "Hormonal changes when falling in love". Psychoneuroendocrinology. 29 (7): 931–6. doi:10.1016/j.psyneuen.2003.08.006. PMID 15177709. S2CID 24651931.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Berg SJ, Wynne-Edwards KE (2001). "Changes in testosterone, cortisol, and estradiol levels in men becoming fathers". Mayo Clinic Proceedings. 76 (1): 582–592. doi:10.4065/76.6.582. PMID 11393496.
- ^ Van Anders S.M. & Watson, N.V. (2006). "Relationship status and testosterone in North American heterosexual and non-heterosexual men and women: Cross-sectional and longitudinal data". Psychoneuroendocrinology. 31 (6): 715–723. doi:10.1016/j.psyneuen.2006.01.008. hdl:2027.42/83924. PMID 16621328. S2CID 22477678.
- ^ "Testosterone and men's marriages". Soc. Forces. 72 (2): 463–477. 1993. doi:10.2307/2579857. JSTOR 2579857.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ "Testosterone and men's marriages". Soc. Forces. 72 (2): 463–477. 1993. doi:10.2307/2579857. JSTOR 2579857.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ "Testosterone and men's marriages". Soc. Forces. 72 (2): 463–477. 1993. doi:10.2307/2579857. JSTOR 2579857.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ Van Anders S.M. & Watson, N.V. (2006). "Relationship status and testosterone in North American heterosexual and non-heterosexual men and women: Cross-sectional and longitudinal data". Psychoneuroendocrinology. 31 (6): 715–723. doi:10.1016/j.psyneuen.2006.01.008. hdl:2027.42/83924. PMID 16621328. S2CID 22477678.
- ^ Van Anders S.M. & Watson N.V. (2007). "Testosterone levels in women and men who are single, in long-distance relationships, or same-city relationships". Hormones and Behaviour. 51: 477–482.
- ^ Marazziti D, Canale D (2004). "Hormonal changes when falling in love". Psychoneuroendocrinology. 29 (7): 931–6. doi:10.1016/j.psyneuen.2003.08.006. PMID 15177709. S2CID 24651931.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Fox, C. A., Ismail, A. A., Love, D.N., Kirkham, K. E., & Loraine, J.A. (1972). "Studies on the relationship between plasma testosterone levels and human sexual activity". J. Endocrinol. 52 (52): 51–58. doi:10.1677/joe.0.0520051. PMID 5061159.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Van Anders S.M. & Watson N.V. (2006). "Menstrual cycle irregularities are associated with testosterone levels in healthy premenopausal women". Am J Hum Biol. 18 (6): 841–4. doi:10.1002/ajhb.20555. hdl:2027.42/83925. PMID 17039468. S2CID 32023452.
- ^ Van Anders S.M. & Watson N.V. (2006). "Menstrual cycle irregularities are associated with testosterone levels in healthy premenopausal women". Am J Hum Biol. 18 (6): 841–4. doi:10.1002/ajhb.20555. hdl:2027.42/83925. PMID 17039468. S2CID 32023452.
- ^ Van Anders S. M. & Dunn, E. J. (2009). "Are gonadal steroids linked with orgasm perceptions and sexual assertiveness in women and men?". Hormones and Behavior. 56 (2): 206–213. doi:10.1016/j.yhbeh.2009.04.007. hdl:2027.42/83876. PMID 19409392. S2CID 14588630.
- ^ Harding, S. M. & Velotta, J. P. (2011). "Comparing the relative amount of testosterone required to restore sexual arousal, motivation, and performance in rats". Hormones and Behavior. 59 (5): 666–673. doi:10.1016/j.yhbeh.2010.09.009. PMID 20920505. S2CID 1577450.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ James, P. J., Nyby, J.G., & Saviolakis, G.A. (2006). "Sexually stimulated testosterone release in male mice (Mus musculus): Roles of genotype and sexual arousal". Hormones and Behavior. 5 (3): 424–431. doi:10.1016/j.yhbeh.2006.05.004. PMID 16828762. S2CID 36436418.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ James, P. J., Nyby, J.G., & Saviolakis, G.A. (2006). "Sexually stimulated testosterone release in male mice (Mus musculus): Roles of genotype and sexual arousal". Hormones and Behavior. 5 (3): 424–431. doi:10.1016/j.yhbeh.2006.05.004. PMID 16828762. S2CID 36436418.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wallen, K. (2001). "Sex and context: hormones and primate sexual motivation". Hormones and Behavior. 40 (2): 339–357. doi:10.1006/hbeh.2001.1696. PMID 11534996. S2CID 2214664.
- ^ Wallen, K. (2001). "Sex and context: hormones and primate sexual motivation". Hormones and Behavior. 40 (2): 339–357. doi:10.1006/hbeh.2001.1696. PMID 11534996. S2CID 2214664.
- ^ Kraemer, H.C., Becker, H.B., Brodie, H.K., Doering, C.H., Moos, R.H., & Hamburg, D.A. (1976). "Orgasmic frequency and plasma testosterone levels in normal human males". Arch. Sex. Behav. 5 (2): 125–132. doi:10.1007/BF01541869. PMID 1275688. S2CID 38283107.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pirke, K.M., Kockott, G., & Dittmar, F. (1974). "Psychosexual stimulation and plasma testosterone in man". Archives of Sexual Behavior. 3 (6): 577–584. doi:10.1007/BF01541140. PMID 4429441. S2CID 43495791.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Miller, S. L. & Maner, J. K. (2010). "Scent of a Woman: Men's Testosterone Responses to Olfactory Ovulation Cues". Psychological Science. 21 (2): 276–283. doi:10.1177/0956797609357733. PMID 20424057. S2CID 18170407.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gangestead, S.W., Thornhill, R., & Garver-Apgar, C. E. (2005). "Adaptations to Ovulation: Implications for Sexual and Social Behavior". Current Directions in Psychological Science. 14 (6): 312–316. doi:10.1111/j.0963-7214.2005.00388.x. S2CID 53074076.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Alexander, G. M. & Sherwin, B. B. (1991). "The association between testosterone, sexual arousal, and selective attention for erotic stimuli in men". Hormones and Behavior. 25 (3): 367–381. doi:10.1016/0018-506X(91)90008-6. PMID 1937428. S2CID 45677152.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hart, B. (1983). "Role of testosterone secretion and penile reflexes in sexual behavior and sperm competition in male rats: A theoretical contribution". Physiology & Behaviour. 31 (6): 823–827. doi:10.1016/0031-9384(83)90279-2. PMID 6665072. S2CID 42155431.
- ^ A.M. Traish, N. Kim, K. Min, R. Munarriz, I. Goldstein (2002). "Role of androgens in female genital sexual arousal: receptor expression, structure, and function". Fertil. Steril. 77: S11-8. doi:10.1016/S0015-0282(02)02978-3. PMID 12007897.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Van Anders, S. M., Hamilton, D. L., Schmidt, N., & Watson, N. V. (2007). "Associations between testosterone secretion and sexual activity in women". Simon Fraser University.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ Goldey, K. L. & Van Anders S.M. (2011). "Sexy thoughts: Effects of sexual cognitions on testosterone, cortisol, and arousal in women". Hormones and Behavior. 59 (5): 754–764. doi:10.1016/j.yhbeh.2010.12.005. hdl:2027.42/83874. PMID 21185838. S2CID 18691358.
- ^ Tuiten, A., Van Honk, J., Koppeschaar, H., Bernaards, C., Thijssen, J., & Verbaten, R. (2000). "Time Course of Effects of Testosterone Administration on Sexual Arousal in Women". Arch Gen Psychiatry. 57 (2): 149–153. doi:10.1001/archpsyc.57.2.149. PMID 10665617.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bolour, S. & Braunstein G. (2005). "Testosterone therapy in women: a review". International Journal of Impotence Research. 17 (5): 399–408. doi:10.1038/sj.ijir.3901334. PMID 15889125. S2CID 6461717.
- ^ Bolour, S. & Braunstein G. (2005). "Testosterone therapy in women: a review". International Journal of Impotence Research. 17 (5): 399–408. doi:10.1038/sj.ijir.3901334. PMID 15889125. S2CID 6461717.
- ^ Sapienza P, Zingales L, Maestripieri D (2009). "Gender differences in financial risk aversion and career choices are affected by testosterone". Proc. Natl. Acad. Sci. U.S.A. 106 (36): 15268–73. Bibcode:2009PNAS..10615268S. doi:10.1073/pnas.0907352106. PMC 2741240. PMID 19706398.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Apicella CL, Dreber A, Campbell B, Gray PB, Hoffman M, Little AC (2008). "Testosterone and financial risk preferences". Evolution and Human Behavior. 29 (6): 384–390. doi:10.1016/j.evolhumbehav.2008.07.001.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Zak PJ; et al. (2009). Aleman, André (ed.). "Testosterone Administration Decreases Generosity in the Ultimatum Game". PLOS ONE. 4 (12): e8330. Bibcode:2009PLoSO...4.8330Z. doi:10.1371/journal.pone.0008330. PMC 2789942. PMID 20016825.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: unflagged free DOI (link) - ^ Wilson JD (2001). "Androgens, androgen receptors, and male gender role behavior". Horm Behav. 40 (2): 358–66. doi:10.1006/hbeh.2001.1684. PMID 11534997. S2CID 20480423.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Cosgrove, KP (2007). "Evolving Knowledge of Sex Differences in Brain Structure, Function and Chemistry". Biol Psychiat. 62 (8): 847–55. doi:10.1016/j.biopsych.2007.03.001. PMC 2711771. PMID 17544382.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Marner L, Nyengaard JR, Tang Y, Pakkenberg B. (2003). Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 462(2):144-52. PMID 12794739
- ^ Testosterone Affects Some Women's Career Choices
- ^ Hogervorst E, Bandelow S, Combrinck M, Smith AD (2004). "Low free testosterone is an independent risk factor for Alzheimer's disease". Exp. Gerontol. 39 (11–12): 1633–9. doi:10.1016/j.exger.2004.06.019. PMID 15582279. S2CID 24803152.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM (2004). "Free testosterone and risk for Alzheimer disease in older men". Neurology. 62 (2): 188–93. doi:10.1212/wnl.62.2.188. PMID 14745052. S2CID 10302839.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Moffat SD, Hampson E (1996). "A curvilinear relationship between testosterone and spatial cognition in humans: possible influence of hand preference". Psychoneuroendocrinology. 21 (3): 323–37. doi:10.1016/0306-4530(95)00051-8. PMID 8817730. S2CID 7135870.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Pike CJ, Rosario ER, Nguyen TV (2006). "Androgens, aging, and Alzheimer's disease". Endocrine. 29 (2): 233–41. doi:10.1385/ENDO:29:2:233. PMID 16785599. S2CID 13852805.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Rosario ER, Chang L, Stanczyk FZ, Pike CJ (2004). "Age-related testosterone depletion and the development of Alzheimer disease". JAMA. 292 (12): 1431–2. doi:10.1001/jama.292.12.1431-b. PMID 15383512.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wright J, Ellis L, Beaver K (2009). Handbook of crime correlates. San Diego: Academic Press. ISBN 978-0-12-373612-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Soma, KK; Scotti, MA; Newman, AE; Charlier, TD; Demas, GE (2008). "Novel mechanisms for neuroendocrine regulation of aggression". Frontiers in Neuroendocrinology. 29 (4): 476–89. doi:10.1016/j.yfrne.2007.12.003. PMID 18280561. S2CID 32650274.
- ^ Rohrmann, S; Sutcliffe, C. G.; Bienstock, J. L.; Monsegue, D; Akereyeni, F; Bradwin, G; Rifai, N; Pollak, M. N.; Agurs-Collins, T; Platz, E. A. (May 2009). "Racial variation in sex steroid hormones and the insulin-like growth factor axis in umbilical cord blood of male neonates". Cancer Epidemiol. Biomarkers Prev. 18 (5): 1484–1491. doi:10.1158/1055-9965.EPI-08-0817. PMC 3012385. PMID 19423525.
- ^ McIntosh, H. (1997). "Why do African-American men suffer more prostate cancer?". JNCI Journal of the National Cancer Institute. 89 (3): 188–189. doi:10.1093/jnci/89.3.188. PMID 9016996.
- ^ Calistro Alvarado, L. (2010). "Population differences in the testosterone levels of young men are associated with prostate cancer disparities in older men". American Journal of Human Biology. 22 (4): 449–455. doi:10.1002/ajhb.21016. PMID 20087895. S2CID 21117845.
- ^ a b de Kruif P (1945). The Male Hormone. New York: Harcourt, Brace.
- ^ Myers JB, Meacham RB (2003). "Androgen Replacement Therapy in the Aging Male". Rev Urol. 5 (4): 216–26. PMC 1508369. PMID 16985841.
- ^ a b Davis SR, Moreau M, Kroll R, Bouchard C, Panay N, Gass M, Braunstein GD, Hirschberg AL, Rodenberg C, Pack S, Koch H, Moufarege A, Studd J (2008). "Testosterone for low libido in postmenopausal women not taking estrogen". N. Engl. J. Med. 359 (19): 2005–17. doi:10.1056/NEJMoa0707302. PMID 18987368.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Testosterone replacement therapy for male aging: ASA position statement". J. Androl. 27 (2): 133–4. 2006. doi:10.1002/j.1939-4640.2006.tb01177.x. PMID 16474019.
- ^ Guay AT, Spark RF, Bansal S, Cunningham GR, Goodman NF, Nankin HR, Petak SM, Perez JB (2003). "American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of male sexual dysfunction: a couple's problem—2003 update" (PDF). Endocr Pract. 9 (1): 77–95. doi:10.4158/EP.9.1.77. PMID 12917096.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Holt EH, Zieve D (2008-03-18). "Testosterone". MedlinePlus Medical Encyclopedia. U.S. National Library of Medicine. Retrieved 2009-07-17.
- ^ "Contraceptive efficacy of testosterone-induced azoospermia in normal men. World Health Organization Task Force on methods for the regulation of male fertility". Lancet. 336 (8721): 955–9. 1990. doi:10.1016/0140-6736(90)92416-F. PMID 1977002. S2CID 25825354.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Traish AM, Saad F, Guay A (2009). "The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance". J. Androl. 30 (1): 23–32. doi:10.2164/jandrol.108.005751. PMID 18772488.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, Grobbee DE, van der Schouw YT (2008). "Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial". JAMA. 299 (1): 39–52. doi:10.1001/jama.2007.51. PMID 18167405.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Cunningham GR (2008-06-25). "Testosterone treatment in aging men". EndocrineToday.com. Retrieved 2009-07-17.
- ^ Gaylis FD, Lin DW, Ignatoff JM, Amling CL, Tutrone RF, Cosgrove DJ. (2005). "Prostate cancer in men using testosterone supplementation". J Urol. 174 (2): 534–538. doi:10.1097/01.ju.0000165166.36280.60. PMID 16006887.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Anabolic Steroid Control Act" (PDF). United States Sentencing Commission. 1990.
- ^ Strahm E, Emery C, Saugy M, Dvorak J, Saudan C (2009). "Detection of testosterone administration based on the carbon isotope ratio profiling of endogenous steroids: international reference populations of professional soccer players". Br J Sports Med. 43 (13): 1041–4. doi:10.1136/bjsm.2009.058669. PMC 2784500. PMID 19549614.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kicman AT, Cowan DA (2009). "Subject-based profiling for the detection of testosterone administration in sport". Drug Test Anal. 1 (1): 22–4. doi:10.1002/dta.14. PMID 20355155.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Pozo OJ, Deventer K, Van Eenoo P, et al. Quantification of testosterone undecanoate in human hair by liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 23: 873–880, 2009.
- ^ Baselt RC (2008). Disposition of Toxic Drugs & Chemicals in Man (8th ed.). Foster City, Calif: Biomedical Publications. pp. 1501–1504. ISBN 978-0-9626523-7-0.
- ^ "Testosterone Information". Drugs.com.
- ^ "Striant Official FDA information, side effects and uses". Drugs.com.
- ^ "AndroGel Official FDA information, side effects and uses". Drugs.com.
- ^ "Testim (patches and gel) medical facts". Drugs.com.
- ^ "Testopel Pellets" (PDF). www.slatepharma.com.
- ^ Waterman MR, Keeney DS (1992). "Genes involved in androgen biosynthesis and the male phenotype". Horm. Res. 38 (5–6): 217–21. doi:10.1159/000182546. PMID 1307739.
- ^ Zuber MX, Simpson ER, Waterman MR (1986). "Expression of bovine 17 alpha-hydroxylase cytochrome P-450 cDNA in nonsteroidogenic (COS 1) cells". Science. 234 (4781): 1258–61. Bibcode:1986Sci...234.1258Z. doi:10.1126/science.3535074. PMID 3535074.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Zouboulis, C. C.; Degitz, K. (2004). "Androgen action on human skin -- from basic research to clinical significance". Experimental Dermatology. 13 Suppl 4: 5–10. doi:10.1111/j.1600-0625.2004.00255.x. PMID 15507105. S2CID 34863608.
- ^ Brooks RV (1975). "Androgens". Clin Endocrinol Metab. 4 (3): 503–20. doi:10.1016/s0300-595x(75)80045-4. PMID 58744.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Payne AH, O'Shaughnessy P (1996). "Structure, function, and regulation of steroidogenic enzymes in the Leydig cell". In Payne AH, Hardy MP, Russell LD (ed.). Leydig Cell. Vienna [Il]: Cache River Press. pp. 260–285. ISBN 0-9627422-7-9.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ Swerdloff RS, Wang C, Bhasin S (1992). "Developments in the control of testicular function". Baillieres Clin. Endocrinol. Metab. 6 (2): 451–83. doi:10.1016/S0950-351X(05)80158-2. PMID 1377467.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Håkonsen LB, Thulstrup AM, Aggerholm AS, Olsen J, Bonde JP, Andersen CY, Bungum M, Ernst EH, Hansen ML, Ernst EH, Ramlau-Hansen CH (2011). "Does weight loss improve semen quality and reproductive hormones? Results from a cohort of severely obese men". Reprod Health. 8: 24. doi:10.1186/1742-4755-8-24. PMC 3177768. PMID 21849026.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B, Wehr E, Zittermann A (2011). "Effect of vitamin D supplementation on testosterone levels in men". Horm. Metab. Res. 43 (3): 223–5. doi:10.1055/s-0030-1269854. PMID 21154195.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Prasad AS, Mantzoros CS, Beck FW, Hess JW, Brewer GJ (1996). "Zinc status and serum testosterone levels of healthy adults". Nutrition. 12 (5): 344–8. doi:10.1016/S0899-9007(96)80058-X. PMID 8875519.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Koehler K, Parr MK, Geyer H, Mester J, Schänzer W (2009). "Serum testosterone and urinary excretion of steroid hormone metabolites after administration of a high-dose zinc supplement". Eur J Clin Nutr. 63 (1): 65–70. doi:10.1038/sj.ejcn.1602899. PMID 17882141. S2CID 26507955.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Schultheiss OC, Campbell KL, McClelland DC (1999). "Implicit power motivation moderates men's testosterone responses to imagined and real dominance success". Horm Behav. 36 (3): 234–41. doi:10.1006/hbeh.1999.1542. PMID 10603287. S2CID 6002474.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Liu PY, Pincus SM, Takahashi PY, Roebuck PD, Iranmanesh A, Keenan DM, Veldhuis JD (2006). "Aging attenuates both the regularity and joint synchrony of LH and testosterone secretion in normal men: analyses via a model of graded GnRH receptor blockade". Am. J. Physiol. Endocrinol. Metab. 290 (1): E34–E41. doi:10.1152/ajpendo.00227.2005. PMID 16339924.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Andersen ML, Tufik S (2008). "The effects of testosterone on sleep and sleep-disordered breathing in men: its bidirectional interaction with erectile function" (PDF). Sleep Med Rev. 12 (5): 365–79. doi:10.1016/j.smrv.2007.12.003. PMID 18519168.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Marin DP, Figueira AJ Junior, Pinto LG (2006). "One session of resistance training may increase serum testosterone and triiodetironine in young men". Medicine & Science in Sports & Exercise. 38 (5): S285. doi:10.1249/00005768-200605001-02108.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hulmi JJ, Ahtiainen JP, Selänne H, Volek JS, Häkkinen K, Kovanen V, Mero AA (2008). "Androgen receptors and testosterone in men—effects of protein ingestion, resistance exercise and fiber type". J. Steroid Biochem. Mol. Biol. 110 (1–2): 130–7. doi:10.1016/j.jsbmb.2008.03.030. PMID 18455389. S2CID 26280370.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Josephs RA, Guinn JS, Harper ML, Askari F (2001). "Liquorice consumption and salivary testosterone concentrations". Lancet. 358 (9293): 1613–4. doi:10.1016/S0140-6736(01)06664-8. PMID 11716893. S2CID 31788670.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Armanini D, Mattarello MJ, Fiore C, Bonanni G, Scaroni C, Sartorato P, Palermo M (2004). "Licorice reduces serum testosterone in healthy women". Steroids. 69 (11–12): 763–6. doi:10.1016/j.steroids.2004.09.005. PMID 15579328. S2CID 23274304.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Akdoğan M, Tamer MN, Cüre E, Cüre MC, Köroğlu BK, Delibaş N (2007). "Effect of spearmint (Mentha spicata Labiatae) teas on androgen levels in women with hirsutism". Phytother Res. 21 (5): 444–7. doi:10.1002/ptr.2074. PMID 17310494. S2CID 21961390.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kumar V, Kural MR, Pereira BM, Roy P (2008). "Spearmint induced hypothalamic oxidative stress and testicular anti-androgenicity in male rats – altered levels of gene expression, enzymes and hormones". Food Chem. Toxicol. 46 (12): 3563–70. doi:10.1016/j.fct.2008.08.027. PMID 18804513.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Grant P (2010). "Spearmint herbal tea has significant anti-androgen effects in polycystic ovarian syndrome. A randomized controlled trial". Phytother Res. 24 (2): 186–8. doi:10.1002/ptr.2900. PMID 19585478. S2CID 206425734.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Randall VA (1994). "Role of 5 alpha-reductase in health and disease". Baillieres Clin. Endocrinol. Metab. 8 (2): 405–31. doi:10.1016/S0950-351X(05)80259-9. PMID 8092979.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Meinhardt U, Mullis PE (2002). "The essential role of the aromatase/p450arom". Semin. Reprod. Med. 20 (3): 277–84. doi:10.1055/s-2002-35374. PMID 12428207.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Trager L (1977). Steroidhormone: Biosynthese, Stoffwechsel, Wirkung (in German). Springer-Verlag. p. 349. ISBN 0-3870-8012-0.
- ^ Hiipakka RA, Liao S (1998). "Molecular mechanism of androgen action". Trends Endocrinol. Metab. 9 (8): 317–24. doi:10.1016/S1043-2760(98)00081-2. PMID 18406296. S2CID 23385663.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ McPhaul MJ, Young M (2001). "Complexities of androgen action". J. Am. Acad. Dermatol. 45 (3 Suppl): S87–94. doi:10.1067/mjd.2001.117429. PMID 11511858.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Breiner M, Romalo G, Schweikert HU (1986). "Inhibition of androgen receptor binding by natural and synthetic steroids in cultured human genital skin fibroblasts". Klin. Wochenschr. 64 (16): 732–7. doi:10.1007/BF01734339. PMID 3762019. S2CID 34846760.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Bratoeff E, Cabeza M, Ramirez E, Heuze Y, Flores E (2005). "Recent advances in the chemistry and pharmacological activity of new steroidal antiandrogens and 5 alpha-reductase inhibitors". Curr. Med. Chem. 12 (8): 927–43. doi:10.2174/0929867053507306. PMID 15853706.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Engel JB, Schally AV (2007). "Drug Insight: clinical use of agonists and antagonists of luteinizing-hormone-releasing hormone". Nat Clin Pract Endocrinol Metab. 3 (2): 157–67. doi:10.1038/ncpendmet0399. PMID 17237842. S2CID 19745821.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Gould DC, Petty R (2000). "The male menopause: does it exist?: For: Some men need investigation and testosterone treatment". West. J. Med. 173 (2): 76–8. doi:10.1136/ewjm.173.2.76. PMC 1070997. PMID 10924412.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Berthold AA (1849). "Transplantation der Hoden" [Transplantation of testis]. Arch. Anat. Physiol. Wissensch. (in German). 16: 42–6.
- ^ Brown-Sequard CE (1889). "The effects produced on man by subcutaneous injections of liquid obtained from the testicles of animals". Lancet. 2 (3438): 105. doi:10.1016/S0140-6736(00)64118-1.
- ^ Gallagher TF, Koch FC (1929). "The testicular horomone". J. Biol. Chem. 84 (2): 495–500. doi:10.1016/S0021-9258(18)77008-7.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ David KG., Dingemanse E, Freud J. Laqueur E (1935). "Über krystallinisches mannliches Hormon aus Hoden (Testosteron) wirksamer als aus harn oder aus Cholesterin bereitetes Androsteron" [On crystalline male hormone from testicles (testosterone) effective as from urine or from cholesterol]. Hoppe Seylers Z Physiol Chem (in German). 233 (5–6): 281. doi:10.1515/bchm2.1935.233.5-6.281.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Butenandt A, Hanisch G (1935). "Umwandlung des Dehydroandrosterons in Androstendiol und Testosterone; ein Weg zur Darstellung des Testosterons aus Cholestrin" [About Testosterone. Conversion of Dehydro-androsterons into androstendiol and testosterone; a way for the structure assignment of testosterone from cholestrol]. Hoppe Seylers Z Physiol Chem (in German). 237 (2): 89. doi:10.1515/bchm2.1935.237.1-3.89.
- ^ a b Freeman ER, Bloom DA, McGuire EJ (2001). "A brief history of testosterone". J. Urol. 165 (2): 371–3. doi:10.1097/00005392-200102000-00004. PMID 11176375.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Butenandt A, Hanisch G (1935). "Uber die Umwandlung des Dehydroandrosterons in Androstenol-(17)-one-(3) (Testosterone); um Weg zur Darstellung des Testosterons auf Cholesterin (Vorlauf Mitteilung). [The conversion of dehydroandrosterone into androstenol-(17)-one-3 (testosterone); a method for the production of testosterone from cholesterol (preliminary communication)]". Chemische Berichte (in German). 68: 1859–1862.
- ^ Ruzicka L, Wettstein A (1935). "Uber die kristallinische Herstellung des Testikelhormons, Testosteron (Androsten-3-ol-17-ol) [The crystalline production of the testicle hormone, testosterone (Androsten-3-ol-17-ol]". Helvetica Chimica Acta (in German). 18: 1264–1275. doi:10.1002/hlca.193501801176.
- ^ Hoberman JM, Yesalis CE (1995). "The history of synthetic testosterone". Sci. Am. 272 (2): 76–81. doi:10.1038/scientificamerican0295-76. PMID 7817189.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Kenyon AT, Knowlton K, Sandiford I, Koch FC, Lotwin,G (1940). "A comparative study of the metabolic effects of testosterone propionate in normal men and women and in eunuchoidism". Endocrinology. 26 (1): 26–45. doi:10.1210/Endo-26-1-26.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Schwarz S, Onken D, Schubert A (1999). "The steroid story of Jenapharm: from the late 1940s to the early 1970s". Steroids. 64 (7): 439–45. doi:10.1016/S0039-128X(99)00003-3. PMID 10443899. S2CID 40156824.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Travison TG, Araujo AB, O'Donnell AB, Kupelian V, McKinlay JB (2007). "A population-level decline in serum testosterone levels in American men". J. Clin. Endocrinol. Metab. 92 (1): 196–202. doi:10.1210/jc.2006-1375. PMID 17062768.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Dindyal S (2007). "The sperm count has been decreasing steadily for many years in Western industrialised countries: Is there an endocrine basis for this decrease?". The Internet Journal of Urology. 2 (1): 1–21.
- ^ Bhasin S (2007). "Secular decline in male reproductive function: Is manliness threatened?". J. Clin. Endocrinol. Metab. 92 (1): 44–5. doi:10.1210/jc.2006-2438. PMID 17209224.
{{cite journal}}: Unknown parameter|month=ignored (help)
