Β-Hydroxy β-methylbutyric acid: Difference between revisions
ce |
re-add a revision of language by Jytdog w/ small copyedit to reflect a recommendation by two sets of review authors |
||
| Line 96: | Line 96: | ||
====Muscle wasting==== |
====Muscle wasting==== |
||
HMB has been used in a number of clinical trials as a treatment for preserving [[lean body mass]] in [[muscle wasting]] conditions, particularly [[sarcopenia]] and during [[bed rest]], and is often employed as an [[adjunct therapy]] in conjunction with [[physical exercise]].<ref name="Molecular Aspects of Medicine 2016 review" /><ref name="Meta-analytic systematic review September 2015" /><ref name="Skeletal muscle crosstalk 2016 review" /><ref name="Skeletal muscle homeostasis 2016 review" /> A growing body of evidence supports the efficacy of HMB as a treatment for reducing, or even reversing, the loss of muscle mass, [[Muscle#Physiology|muscle function]], and [[Muscle#Strength|muscle strength]] that occurs in hypercatabolic disease states such as [[cancer cachexia]];<ref name="Molecular Aspects of Medicine 2016 review" /><ref name="Skeletal muscle crosstalk 2016 review">{{cite journal | vauthors = Argilés JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Mañas L | title = Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease | journal = J. Am. Med. Dir. Assoc. | volume = | issue = | pages = | date = June 2016 | pmid = 27324808 | doi = 10.1016/j.jamda.2016.04.019 | url = http://www.jamda.com/article/S1525-8610(16)30113-X/fulltext | quote = Studies suggest dietary protein and leucine or its metabolite b-hydroxy b-methylbutyrate (HMB) can improve muscle function, in turn improving functional performance. ... These have identified the leucine metabolite β-hydroxy β-methylbutyrate (HMB) as a potent stimulator of protein synthesis as well as an inhibitor of protein breakdown in the extreme case of cachexia.<sup>65, 72, 76, 77, 78, 79, 80, 81, 82, 83, 84</sup> A growing body of evidence suggests HMB may help slow, or even reverse, the muscle loss experienced in sarcopenia and improve measures of muscle strength.<sup>44, 65, 72, 76, 77, 78, 79, 80, 81, 82, 83, 84</sup> However, dietary leucine does not provide a large amount of HMB: only a small portion, as little as 5%, of catabolized leucine is metabolized into HMB.<sup>85</sup> Thus, although dietary leucine itself can lead to a modest stimulation of protein synthesis by producing a small amount of HMB, direct ingestion of HMB more potently affects such signaling, resulting in demonstrable muscle mass accretion.<sup>71, 80</sup> Indeed, a vast number of studies have found that supplementation of HMB to the diet may reverse some of the muscle loss seen in sarcopenia and in hypercatabolic disease.<sup>65, 72, 83, 86, 87</sup> The overall treatment of muscle atrophy should include dietary supplementation with HMB, although the optimal dosage for each condition is still under investigation.<sup>68</sup> ...<br />[http://www.jamda.com/cms/attachment/2060482578/2062671998/gr4_lrg.jpg Figure 4: Treatments for sarcopenia.] It is currently recommended that patients at risk of or suffering from sarcopenia consume a diet high in protein, engage in resistance exercise, and take supplements of the leucine metabolite HMB.}}</ref><ref name="Nutrition supplements for athletes 2014 review">{{cite journal | vauthors = Mullin GE | title = Nutrition supplements for athletes: potential application to malnutrition | journal = Nutr. Clin. Pract. | volume = 29 | issue = 1 | pages = 146–147 | date = February 2014 | pmid = 24336486 | doi = 10.1177/0884533613516130 | quote = There are a number of nutrition products on the market that are touted to improve sports performance. HMB appears to be the most promising and to have clinical applications to improve muscle mass and function. Continued research using this nutraceutical to prevent and/or improve malnutrition in the setting of muscle wasting is warranted.}}</ref> consequently, |
HMB has been used in a number of clinical trials as a treatment for preserving [[lean body mass]] in [[muscle wasting]] conditions, particularly [[sarcopenia]] and during [[bed rest]], and is often employed as an [[adjunct therapy]] in conjunction with [[physical exercise]].<ref name="Molecular Aspects of Medicine 2016 review" /><ref name="Meta-analytic systematic review September 2015" /><ref name="Skeletal muscle crosstalk 2016 review" /><ref name="Skeletal muscle homeostasis 2016 review" /> A growing body of evidence supports the efficacy of HMB as a treatment for reducing, or even reversing, the loss of muscle mass, [[Muscle#Physiology|muscle function]], and [[Muscle#Strength|muscle strength]] that occurs in hypercatabolic disease states such as [[cancer cachexia]];<ref name="Molecular Aspects of Medicine 2016 review" /><ref name="Skeletal muscle crosstalk 2016 review">{{cite journal | vauthors = Argilés JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Mañas L | title = Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease | journal = J. Am. Med. Dir. Assoc. | volume = | issue = | pages = | date = June 2016 | pmid = 27324808 | doi = 10.1016/j.jamda.2016.04.019 | url = http://www.jamda.com/article/S1525-8610(16)30113-X/fulltext | quote = Studies suggest dietary protein and leucine or its metabolite b-hydroxy b-methylbutyrate (HMB) can improve muscle function, in turn improving functional performance. ... These have identified the leucine metabolite β-hydroxy β-methylbutyrate (HMB) as a potent stimulator of protein synthesis as well as an inhibitor of protein breakdown in the extreme case of cachexia.<sup>65, 72, 76, 77, 78, 79, 80, 81, 82, 83, 84</sup> A growing body of evidence suggests HMB may help slow, or even reverse, the muscle loss experienced in sarcopenia and improve measures of muscle strength.<sup>44, 65, 72, 76, 77, 78, 79, 80, 81, 82, 83, 84</sup> However, dietary leucine does not provide a large amount of HMB: only a small portion, as little as 5%, of catabolized leucine is metabolized into HMB.<sup>85</sup> Thus, although dietary leucine itself can lead to a modest stimulation of protein synthesis by producing a small amount of HMB, direct ingestion of HMB more potently affects such signaling, resulting in demonstrable muscle mass accretion.<sup>71, 80</sup> Indeed, a vast number of studies have found that supplementation of HMB to the diet may reverse some of the muscle loss seen in sarcopenia and in hypercatabolic disease.<sup>65, 72, 83, 86, 87</sup> The overall treatment of muscle atrophy should include dietary supplementation with HMB, although the optimal dosage for each condition is still under investigation.<sup>68</sup> ...<br />[http://www.jamda.com/cms/attachment/2060482578/2062671998/gr4_lrg.jpg Figure 4: Treatments for sarcopenia.] It is currently recommended that patients at risk of or suffering from sarcopenia consume a diet high in protein, engage in resistance exercise, and take supplements of the leucine metabolite HMB.}}</ref><ref name="Nutrition supplements for athletes 2014 review">{{cite journal | vauthors = Mullin GE | title = Nutrition supplements for athletes: potential application to malnutrition | journal = Nutr. Clin. Pract. | volume = 29 | issue = 1 | pages = 146–147 | date = February 2014 | pmid = 24336486 | doi = 10.1177/0884533613516130 | quote = There are a number of nutrition products on the market that are touted to improve sports performance. HMB appears to be the most promising and to have clinical applications to improve muscle mass and function. Continued research using this nutraceutical to prevent and/or improve malnutrition in the setting of muscle wasting is warranted.}}</ref> consequently, the authors of two 2016 reviews of the clinical evidence recommended that the prevention and treatment of sarcopenia and muscle wasting in general include supplementation with HMB, regular [[resistance exercise]], and consumption of a [[high-protein diet]].<ref name="Molecular Aspects of Medicine 2016 review" /><ref name="Skeletal muscle crosstalk 2016 review" /> Based upon a [[meta-analysis]] of seven [[randomized controlled trial]]s that was published in 2015, HMB supplementation has efficacy as a treatment for preserving lean muscle mass in older adults.{{#tag:ref|The estimated standard mean difference [[effect size]] for the increase in muscle mass in the HMB [[Treatment and control groups|treatment groups relative to controls]] was {{convert|0.352|kg|lbs}} with a [[95% confidence interval]] of {{convert|0.11|–|0.594|kg|lb}}.<ref name="Meta-analytic systematic review September 2015" /> The studies included in the meta-analysis had durations of 2–12 months and the majority of studies lasted 2–3 months.<ref name="Meta-analytic systematic review September 2015" />|group="note"}}<ref name="Meta-analytic systematic review September 2015">{{cite journal | vauthors = Wu H, Xia Y, Jiang J, Du H, Guo X, Liu X, Li C, Huang G, Niu K | title = Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis | journal = Arch. Gerontol. Geriatr. | volume = 61 | issue = 2 | pages = 168–175 | date = September 2015 | pmid = 26169182 | doi = 10.1016/j.archger.2015.06.020 | quote = RESULTS: A total of seven randomized controlled trials were included, in which 147 older adults received HMB intervention and 140 were assigned to control groups. The meta-analysis showed greater muscle mass gain in the intervention groups compared with the control groups (standard mean difference=0.352kg; 95% confidence interval: 0.11, 0.594; Z value=2.85; P=0.004). There were no significant fat mass changes between intervention and control groups (standard mean difference=-0.08kg; 95% confidence interval: -0.32, 0.159; Z value=0.66; P=0.511).<br />CONCLUSION: Beta-hydroxy-beta-methylbutyrate supplementation contributed to preservation of muscle mass in older adults. HMB supplementation may be useful in the prevention of muscle atrophy induced by bed rest or other factors. Further studies are needed to determine the precise effects of HMB on muscle strength and physical function in older adults.}}</ref> HMB does not appear to significantly affect fat mass in older adults.<ref name="Meta-analytic systematic review September 2015" /> {{As of|2015}}, more research is needed to determine the precise effects on [[Muscle#Strength|muscle strength]] and function in this age group.<ref name="Meta-analytic systematic review September 2015" /> |
||
As a treatment for muscle wasting, it is usually taken as a single 3 gram dose, once per day.<ref name="Molecular Aspects of Medicine 2016 review" /> A more optimal dosing regimen is one 1 gram dose, three times a day, since this ensures elevated [[blood plasma|plasma]] concentrations of HMB throughout the day;<ref name="Molecular Aspects of Medicine 2016 review" /> however, {{as of|June 2016|lc=y}} the best dosing regimen for each condition (i.e., bed rest, cachexia, sarcopenia, etc) is still being investigated.<ref name="Skeletal muscle crosstalk 2016 review" /> |
As a treatment for muscle wasting, it is usually taken as a single 3 gram dose, once per day.<ref name="Molecular Aspects of Medicine 2016 review" /> A more optimal dosing regimen is one 1 gram dose, three times a day, since this ensures elevated [[blood plasma|plasma]] concentrations of HMB throughout the day;<ref name="Molecular Aspects of Medicine 2016 review" /> however, {{as of|June 2016|lc=y}} the best dosing regimen for each condition (i.e., bed rest, cachexia, sarcopenia, etc) is still being investigated.<ref name="Skeletal muscle crosstalk 2016 review" /> |
||
Revision as of 03:12, 20 August 2016
 | |
 Top: β-Hydroxy β-methylbutyric acid Bottom: Calcium hydroxymethylbutyrate | |
| Clinical data | |
|---|---|
| Other names | Conjugate acid form: β-Hydroxyisovaleric acid 3-Hydroxyisovaleric acid Conjugate base form: Hydroxymethylbutyrate |
| Routes of administration | Oral (by mouth) |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolites | HMB-CoA, HMG-CoA, mevalonate, cholesterol, acetoacetyl-CoA, acetyl-CoA |
| Onset of action | HMB-FA: 30–60 minutes[1] HMB-Ca: 1–2 hours[1] |
| Elimination half-life | HMB-FA: 3 hours[1] HMB-Ca: 2.5 hours[1] |
| Excretion | Renal (10–40% excreted)[1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.128.078 |
| Chemical and physical data | |
| Formula | C5H10O3 |
| Molar mass | 118.131 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | Less than −32 °C[3] |
| Boiling point | 128 °C (262 °F) at 7 mmHg[3] |
| |
| |
| (verify) | |
β-Hydroxy β-methylbutyric acid (HMB), otherwise known as its conjugate base, β-hydroxy β-methylbutyrate (hydroxymethylbutyrate, HMB), is a dietary supplement and naturally occurring metabolite in humans that has been studied in clinical trials as a treatment for muscle wasting conditions and by athletes as a performance-enhancing substance.[4][5][6] In clinical research, HMB has been shown to have efficacy for inhibiting muscle wasting when used in addition to physical exercise.[4][6][7] It is also used by athletes to increase exercise-induced gains in muscle size, muscle strength, and lean body mass, reduce exercise-induced muscle damage, and speed recovery from high-intensity exercise.[1][4][5][8][9] There appear to be no issues with safety from long-term use as a nutritional supplement in young adults or older adults.[1][5][10]
HMB is a metabolite of L-leucine that is produced in the body through oxidation of the ketoacid of L-leucine (α-ketoisocaproic acid).[1][11] Since only a small fraction of L-leucine is metabolized into HMB, pharmacologically active concentrations of the compound in blood plasma and muscle tissue can only be achieved by supplementing HMB directly.[1][7][12] A healthy adult weighing 70 kilograms (150 lb) produces 0.2–0.4 grams per day depending upon the amount of L-leucine in their diet, while supplemental HMB is usually taken in doses of 3–6 grams per day.[10] HMB is sold as a dietary supplement in the free acid form and as a monohydrated calcium salt of the conjugate base (β-hydroxy β-methylbutyrate) at a cost of about US$30–50 per month when taking 3 grams per day.[1][4][13] HMB is also contained in several nutritional products marketed by Abbot Laboratories (e.g., certain formulations of Ensure, Juven, and Myoplex),[14] and is present in small amounts in certain foods, such as alfalfa, asparagus, avocados, cauliflower, citrus fruits (e.g., grapefruit), catfish, and milk.[15][16][17]
The effects of HMB on human skeletal muscle were first discovered by Steven L. Nissen at Iowa State University in the mid-1990s.[14][18] As of 2015[update], HMB was not tested for or banned by any athletic organization in the United States or internationally;[5] it is allowed by both the National Collegiate Athletic Association (NCAA) and the World Anti-Doping Agency (WADA) both in and outside of competition.[5][19][20] An NCAA study from 2006 found that 1.9% of college student athletes used HMB as a dietary supplement and the use of HMB among these athletes appears to be increasing.[5][21]
Uses
Clinical research
Muscle wasting
HMB has been used in a number of clinical trials as a treatment for preserving lean body mass in muscle wasting conditions, particularly sarcopenia and during bed rest, and is often employed as an adjunct therapy in conjunction with physical exercise.[4][6][7][22] A growing body of evidence supports the efficacy of HMB as a treatment for reducing, or even reversing, the loss of muscle mass, muscle function, and muscle strength that occurs in hypercatabolic disease states such as cancer cachexia;[4][7][23] consequently, the authors of two 2016 reviews of the clinical evidence recommended that the prevention and treatment of sarcopenia and muscle wasting in general include supplementation with HMB, regular resistance exercise, and consumption of a high-protein diet.[4][7] Based upon a meta-analysis of seven randomized controlled trials that was published in 2015, HMB supplementation has efficacy as a treatment for preserving lean muscle mass in older adults.[note 1][6] HMB does not appear to significantly affect fat mass in older adults.[6] As of 2015[update], more research is needed to determine the precise effects on muscle strength and function in this age group.[6]
As a treatment for muscle wasting, it is usually taken as a single 3 gram dose, once per day.[4] A more optimal dosing regimen is one 1 gram dose, three times a day, since this ensures elevated plasma concentrations of HMB throughout the day;[4] however, as of June 2016[update] the best dosing regimen for each condition (i.e., bed rest, cachexia, sarcopenia, etc) is still being investigated.[7]
Enhancing performance
When combined with an appropriate exercise program, dietary supplementation with HMB dose-dependently augments gains in muscle hypertrophy (i.e., the size of a muscle),[1][5][6] muscle strength,[4][5][9] and lean body mass,[4][5][9] reduces exercise-induced skeletal muscle damage,[note 2][5][6][9] and may expedite recovery from high-intensity exercise.[1][5] HMB produces these effects in part by stimulating myofibrillar muscle protein synthesis and inhibiting muscle protein breakdown through various mechanisms, including activation of mechanistic target of rapamycin complex 1 (mTORC1) and inhibition of the proteasome in skeletal muscles.[4][22]
The inhibition of exercise-induced skeletal muscle damage by HMB is affected by the time that it is used relative to exercise.[1][5] The greatest reduction in skeletal muscle damage from a single bout of exercise has been shown to occur when HMB-Ca is ingested 1–2 hours prior to exercise or HMB-FA is ingested 30–60 minutes prior to exercise.[1]
As of 2015[update], HMB was not tested for or banned by any sporting organization in the United States or internationally;[5] it is allowed by both the NCAA and WADA both in and outside of competition.[5][19][20] An NCAA study from 2006 found that 1.9% of college student athletes used HMB as a dietary supplement and the use of HMB among these athletes appears to be increasing.[5][21]
Available forms
HMB is available as an over-the-counter dietary supplement in the free acid form, β-hydroxy β-methylbutyric acid (HMB-FA), and as a monohydrated calcium salt of the conjugate base, calcium β-hydroxy β-methylbutyrate monohydrate (HMB-Ca).[13] HMB is also contained in several nutritional products marketed by Abbot Laboratories (e.g., certain formulations of Ensure, Juven, and Myoplex),[14] and is present in insignificant quantities in certain foods, such as alfalfa, asparagus, avocados, cauliflower, grapefruit, catfish, and milk.[15][16][17]
Side effects
The safety profile of HMB in adult humans has been well-established by medical reviews looking at a combination of randomized controlled trials in humans as well as extensive animal testing on laboratory rats and certain livestock (i.e., pigs, broiler chickens, and turkeys).[4][5][10][17] In humans, no adverse effects in young adults or older adults have been reported when HMB-Ca is taken in doses of 3 grams per day for up to a year.[4][5][10] Studies on young adults taking 6 grams of HMB-Ca per day for up to two months have also reported no adverse effects.[10] There is limited data on the safety of supplemental HMB in humans who are younger than 18 years old;[1] however, studies with supplemental HMB on young, growing rats and livestock have reported no adverse effects based upon clinical chemistry or observable characteristics.[1][17]
No clinical testing with supplemental HMB has been conducted on pregnant women; however, two animal studies have examined the effects of HMB supplementation in pregnant pigs on the offspring and reported no adverse effects on the fetus.[17]
Pharmacology
• PA: phosphatidic acid
• mTOR: mechanistic target of rapamycin
• AMP: adenosine monophosphate
• ATP: adenosine triphosphate
• AMPK: AMP-activated protein kinase
• PGC‐1α: peroxisome proliferator-activated receptor gamma coactivator-1α
• S6K1: p70S6 kinase
• 4EBP1: eukaryotic translation initiation factor 4E-binding protein 1
• eIF4E: eukaryotic translation initiation factor 4E
• RPS6: ribosomal protein S6
• eEF2: eukaryotic elongation factor 2
• RE: resistance exercise; EE: endurance exercise
• Myo: myofibrillar; Mito: mitochondrial
• AA: amino acids
• ↑ represents activation
• Τ represents inhibition
Pharmacodynamics
As of May 2016[update], components of the signaling cascade that mediates the HMB-induced increase in human skeletal muscle protein synthesis have been identified in vivo.[6][25] Similar to L-leucine,[note 3] HMB has been shown to increase protein synthesis in human skeletal muscle via the phosphorylation of mTOR and subsequent activation of mTORC1, which leads to protein biosynthesis in the ribosome via phosphorylation of mTORC1's immediate targets (the p70S6 kinase and the translation repressor protein 4EBP1).[22][25][27] Chronic supplementation with HMB for one month in rats has also been shown to increase growth hormone and IGF-1 signaling through their associated receptors in certain non-muscle tissues via an unknown mechanism, in turn promoting protein synthesis through increased mTOR phosphorylation.[1][4][17] As of April 2016[update], it is not clear if long-term supplementation with HMB in humans produces a similar increase in growth hormone and IGF-1 signaling in skeletal muscle or any other tissues.[1][4]
As of May 2016[update], the signaling cascade that mediates the HMB-induced reduction in muscle protein breakdown has not been identified in living humans, although it is well-established that it attenuates proteolysis in vivo.[6][25] Unlike L-leucine,[note 4] HMB attenuates muscle protein breakdown in an insulin-independent manner in humans.[25] HMB is believed to reduce muscle protein breakdown in humans by inhibiting the 19S and 20S subunits of the ubiquitin–proteasome system in skeletal muscle and by inhibiting apoptosis of myonuclei in muscle cells via unidentified mechanisms.[4][25][27]
Based upon animal studies, HMB appears to be metabolized into cholesterol within skeletal muscle, thereby enhancing the integrity and function of the muscle cell membrane.[9][12][17] In addition, the effects of HMB on muscle protein metabolism may also facilitate stabilization of the muscle cell membrane.[17] It has been suggested that the observed HMB-induced reduction in the plasma concentration of muscle damage biomarkers (i.e., muscle enzymes such as creatine kinase and lactate dehydrogenase) in humans following intense exercise is mediated by the enhancement of the muscle cell membrane function.[note 2][17]
HMB has been shown to stimulate the proliferation, differentiation, and fusion of human myosatellite cells in vitro, which potentially increases the regenerative capacity of skeletal muscle, by increasing the protein expression of certain myogenic regulatory factors (e.g., myoD and myogenin) and gene transcription factors (e.g., MEF2).[1][10][28] HMB-induced human myosatellite cell proliferation in vitro is mediated through phosphorylation of the mitogen-activated protein kinases ERK1 and ERK2.[10][17][28] HMB-induced human myosatellite differentiation and accelerated fusion of myosatellite cells into muscle tissue in vitro is mediated through phosphorylation of the protein kinase Akt.[10][17][28]
Pharmacokinetics

The free acid (HMB-FA) and monohydrated calcium salt (HMB-Ca) forms of HMB have different pharmacokinetics.[1][13] HMB-FA is more readily absorbed into the bloodstream and has a longer elimination half-life (3 hours) relative to HMB-Ca (2.5 hours).[1][13] The plasma clearance of HMB-FA, which reflects tissue uptake and utilization, is roughly 25–40% higher than the clearance of HMB-Ca as well.[1][13] The fraction of an ingested dose that is excreted in urine does not differ between the two forms.[1]
After ingestion, HMB-Ca is converted to β-hydroxy β-methylbutyrate following dissociation of the calcium moiety in the gut.[1] When the HMB-Ca dosage form is ingested, the magnitude and time at which the peak plasma concentration of HMB occurs depends on the dose and concurrent food intake.[1] Higher HMB-Ca doses increase the rate of absorption, resulting in a peak plasma HMB level (Cmax) that is disproportionately greater than expected of a linear dose-response relationship and which occurs sooner relative to lower doses.[note 5][1] Consumption of HMB-Ca with sugary substances slows the rate of HMB absorption, resulting in a lower peak plasma HMB level that occurs later.[note 5][1]
HMB is eliminated via the kidneys, with roughly 10–40% of an ingested dose being excreted unchanged in urine.[1][2] The remaining 60–90% of the dose is retained in tissues or excreted as HMB metabolites.[1][2] The fraction of a given dose of HMB that is excreted unchanged in urine increases with the dose.[note 6][1]
Biosynthesis

HMB is synthesized in the human body through the metabolism of L-leucine, a branched-chain amino acid.[29] In healthy individuals, approximately 60% of dietary L-leucine is metabolized after several hours, with roughly 5% (2–10% range) of dietary L-leucine being converted to HMB.[2][4][29] Around 40% of dietary L-leucine is converted to acetyl-CoA, which is subsequently used in the synthesis of other compounds.[29]
The vast majority of L-leucine metabolism is initially catalyzed by the branched-chain amino acid aminotransferase enzyme, producing α-ketoisocaproate (α-KIC).[2][29] α-KIC is mostly metabolized by the mitochondrial enzyme branched-chain α-ketoacid dehydrogenase, which converts it to isovaleryl-CoA.[2][29] Isovaleryl-CoA is subsequently metabolized by isovaleryl-CoA dehydrogenase and converted to β-methylcrotonoyl-CoA (MC-CoA), which is used in the synthesis of acetyl-CoA and other compounds.[29] During biotin deficiency, HMB can be synthesized from MC-CoA via enoyl-CoA hydratase and an unknown thioesterase enzyme,[30][31][32] which convert MC-CoA into HMB-CoA and HMB-CoA into HMB respectively.[32] A relatively small amount of α-KIC is metabolized in the liver by the cytosolic enzyme 4-hydroxyphenylpyruvate dioxygenase (KIC dioxygenase), which converts α-KIC to HMB.[2][29][33] In healthy individuals, this minor pathway – which involves the conversion of L-leucine to α-KIC and then HMB – is the predominant route of HMB synthesis.[2][29]
A small fraction of L-leucine metabolism – less than 5% in all tissues except the testes where it accounts for about 33% – is initially catalyzed by leucine aminomutase, producing β-leucine, which is subsequently metabolized into β-ketoisocaproate (β-KIC), β-ketoisocaproyl-CoA, and then acetyl-CoA by a series of uncharacterized enzymes.[29][34] HMB could be produced via certain metabolites that are generated along this pathway, but as of 2015[update] the associated enzymes and reactions involved are not known.[29]
Metabolism
The metabolism of HMB is initially catalyzed by an uncharacterized enzyme which converts it to HMB-CoA.[29][31] HMB-CoA is metabolized by either enoyl-CoA hydratase or another uncharacterized enzyme, producing MC-CoA or hydroxymethylglutaryl-CoA (HMG-CoA) respectively.[2][29] MC-CoA is then converted by the enzyme methylcrotonyl-CoA carboxylase to methylglutaconyl-CoA (MG-CoA), which is subsequently converted to HMG-CoA by methylglutaconyl-CoA hydratase.[2][29][34] HMG-CoA is then cleaved into acetyl-CoA and acetoacetate by HMG-CoA lyase or used in the production of cholesterol via the mevalonate pathway.[2][29]
Physical and chemical properties
β-Hydroxy β-methylbutyric acid and β-hydroxy β-methylbutyrate are structural analogs of butyric acid and butyrate that have a hydroxy group and methyl group attached to the beta carbon of these compounds.[35][36] By extension, β-hydroxybutyric acid and β-methylbutyric acid are also parent compounds of the free acid form of HMB.[35][36] β-Hydroxy β-methylbutyric acid is the conjugate acid of β-hydroxy β-methylbutyrate, while β-hydroxy β-methylbutyrate is the conjugate base of β-hydroxy β-methylbutyric acid.[36][37] Physically, at room temperature, pure HMB-FA occurs as a colorless, transparent liquid.[38][39]
Synthesis
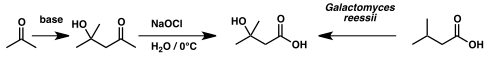
β-Hydroxy-β-methylbutyric acid can be prepared by the oxidation of diacetone alcohol with sodium hypochlorite (NaOCl), more commonly known as bleach.[40][41] Diacetone alcohol in turn may be prepared through the aldol condensation of acetone. Alternatively HMB can be prepared through microbial oxidation of β-methylbutyric acid by the fungus Galactomyces reessii.[42]
Synthesis of β-hydroxy β-methylbutyric acid
|
Detection in body fluids
| Biofluid | Age group | Concentration | Sources | ||
|---|---|---|---|---|---|
| Mean | Range | Units | |||
| Blood | Adults (18+) | 4.0 | 0–10.0 | μM | [35] |
| CSF | Adults (18+) | 4.0 | 2.0–6.0 | μM | [35] |
| Breast milk | Adults (18+) | – | 42–164 | μg/L | [11] |
| Urine | Adults (18+) | – | 3.2–25.0 | μmol/mmol creatinine | [35] |
| Urine | Children (1–13) | – | 0–64.4 | μmol/mmol creatinine | [35] |
Endogenously synthesized HMB has been detected and quantified in several human biofluids using nuclear magnetic resonance spectroscopy (NMR), liquid chromatography–mass spectrometry (LC–MS), and gas chromatography–mass spectrometry (GC–MS) methods.[11][35] In the blood plasma and cerebrospinal fluid (CSF) of healthy adults, the average molar concentration of HMB has been quantified at 4.0 μM.[35] In the urine of healthy individuals of any age, the excreted urinary concentration of HMB has been quantified in a range of 0–68 μmol/mmol creatinine.[35] In the breast milk of healthy lactating women, HMB and L-leucine have been quantified in ranges of 42–164 μg/L and 2.1–88.5 mg/L.[11] In comparison, HMB has been detected and quantified in the milk of healthy cows at a concentration of <20–29 μg/L.[16] This concentration is far too low to be an adequate dietary source of HMB, but milk products could be fortified with HMB to confer benefits to skeletal muscle.[16]
In a study where participants consumed 2.42 grams of pure HMB-FA while fasting, the average plasma HMB concentration increased from a basal level of 5.1 μM to 408 μM after 30 minutes.[25] At 150 minutes post-ingestion, the average plasma HMB concentration among participants was quantified at 275 μM.[25]
Abnormal HMB concentrations in urine and blood plasma have been noted in several disease states where it may serve as a diagnostic biomarker, particularly in the case of metabolic disorders.[35] The following table lists some of these disorders along with the associated HMB concentrations detected in urine or blood plasma.[35]
| Medical condition[note 7] | Biofluid | Age group | Concentration | Sources | ||
|---|---|---|---|---|---|---|
| Mean | Range | Units | ||||
| Biotinidase deficiency† | Blood | Adults (18+) | 9.5 | 0–19.0 | μM | [35] |
| Biotinidase deficiency† | Blood | Children (1–13) | 88.0 | 10.0–166.0 | μM | [35] |
| Biotinidase deficiency† | Urine | Children (1–13) | 275.0 | 50.0–500.0 | μmol/mmol creatinine | [35] |
| 3-Methylglutaconic aciduria (Type I)† | Urine | Children (1–13) | 200.0 | 150.0–250.0 | μmol/mmol creatinine | [35] |
| Eosinophilic esophagitis | Urine | Children (1–13) | 247.4 | 0–699.4 | μmol/mmol creatinine | [35] |
| Gastroesophageal reflux disease | Urine | Children (1–13) | 119.8 | 5.5–234.0 | μmol/mmol creatinine | [35] |
| HMG-CoA lyase deficiency† | Urine | Children (1–13) | 2030.0 | 60.0–4000.0 | μmol/mmol creatinine | [35] |
| MC-CoA carboxylase deficiency† | Urine | Children (1–13) | 30350.0 | 1700.0–59000.0 | μmol/mmol creatinine | [35] |
Notes
- ^ The estimated standard mean difference effect size for the increase in muscle mass in the HMB treatment groups relative to controls was 0.352 kilograms (0.78 lb) with a 95% confidence interval of 0.11–0.594 kilograms (0.24–1.31 lb).[6] The studies included in the meta-analysis had durations of 2–12 months and the majority of studies lasted 2–3 months.[6]
- ^ a b The effect of HMB on skeletal muscle damage has been assessed in studies on humans using four different biomarkers of muscle damage or protein breakdown: serum creatine kinase, serum lactate dehydrogenase, urinary urea nitrogen, and urinary 3-methylhistidine.[1][5][9] When exercise intensity and volume are sufficient to cause skeletal muscle damage, such as during long-distance running or progressive overload, HMB supplementation has been demonstrated to attenuate the rise in these biomarkers by 20–60%.[1][5]
- ^ Approximately equal doses of pure HMB-FA (2.42 grams) and leucine (3.42 grams) do not produce statistically distinguishable anabolic effects, as measured by the fractional synthesis of myofibrillar proteins, in the skeletal muscle of living humans.[25][26] At 150 minutes post-ingestion, these doses of HMB-FA and leucine increased muscle protein synthesis by ∼70% and ∼110% respectively in one study.[25][26]
- ^ At 150 minutes post-ingestion, a 2.42 gram dose of pure HMB-FA decreased skeletal muscle protein breakdown in living humans by 57% in one study.[25][26] The effect of leucine on muscle protein breakdown is entirely dependent upon insulin secretion and consequently was not measured in the same study.[25] By comparison, the insulin-dependent reduction in muscle protein breakdown following an entire meal that contains leucine and carbohydrates is ~50% on average.[25]
- ^ a b In one study, ingestion of a 1 gram dose of HMB-Ca by healthy volunteers produced a peak plasma HMB level of 120 nmol/ml at 2 hours following ingestion, while ingestion of a 3 gram dose of HMB-Ca produced a peak plasma HMB level of 487 nmol/ml at 1 hour following ingestion.[1]
Consumption of 3 grams of HMB-Ca with 75 grams of glucose resulted in a lower peak plasma HMB level of 352 nmol/ml which occurred later at 2 hours following ingestion.[1] - ^ In one study, ingestion of a 1 gram and 3 gram HMB dose resulted in the excretion of 14% and 28% of the dose as HMB in urine, respectively.[1]
- ^ A † indicates that the medical condition is a metabolic disorder.
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, Wilborn C, Kalman DS, Stout JR, Hoffman JR, Ziegenfuss TN, Lopez HL, Kreider RB, Smith-Ryan AE, Antonio J (February 2013). "International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB)". J. Int. Soc. Sports. Nutr. 10 (1): 6. doi:10.1186/1550-2783-10-6. PMC 3568064. PMID 23374455.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b 3-OH-isovaleric acid. Royal Society of Chemistry. Retrieved 10 August 2016.
Experimental Boiling Point: ... 128 °C / 7 mm ...
MP (exp database): <-32 deg C{{cite encyclopedia}}:|work=ignored (help) - ^ a b c d e f g h i j k l m n o p q r Brioche T, Pagano AF, Py G, Chopard A (April 2016). "Muscle wasting and aging: Experimental models, fatty infiltrations, and prevention". Mol. Aspects Med. doi:10.1016/j.mam.2016.04.006. PMID 27106402.
In conclusion, HMB treatment clearly appears to be a safe potent strategy against sarcopenia, and more generally against muscle wasting, because HMB improves muscle mass, muscle strength, and physical performance. It seems that HMB is able to act on three of the four major mechanisms involved in muscle deconditioning (protein turnover, apoptosis, and the regenerative process), whereas it is hypothesized to strongly affect the fourth (mitochondrial dynamics and functions). Moreover, HMB is cheap (~30– 50 US dollars per month at 3 g per day) and may prevent osteopenia (Bruckbauer and Zemel, 2013; Tatara, 2009; Tatara et al., 2007, 2008, 2012) and decrease cardiovascular risks (Nissen et al., 2000). For all these reasons, HMB should be routinely used in muscle-wasting conditions especially in aged people. ... 3 g of CaHMB taken three times a day (1 g each time) is the optimal posology, which allows for continual bioavailability of HMB in the body (Wilson et al., 2013).
- ^ a b c d e f g h i j k l m n o p q r s Momaya A, Fawal M, Estes R (April 2015). "Performance-enhancing substances in sports: a review of the literature". Sports Med. 45 (4): 517–531. doi:10.1007/s40279-015-0308-9. PMID 25663250.
Wilson et al. [91] demonstrated that when non-resistance trained males received HMB pre-exercise, the rise of lactate dehydrogenase (LDH) levels reduced, and HMB tended to decrease soreness. Knitter et al. [92] showed a decrease in LDH and creatine phosphokinase (CPK), a byproduct of muscle breakdown, by HMB after a prolonged run. ... The utility of HMB does seem to be affected by timing of intake prior to workouts and dosage [97].
- ^ a b c d e f g h i j k l Wu H, Xia Y, Jiang J, Du H, Guo X, Liu X, Li C, Huang G, Niu K (September 2015). "Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis". Arch. Gerontol. Geriatr. 61 (2): 168–175. doi:10.1016/j.archger.2015.06.020. PMID 26169182.
RESULTS: A total of seven randomized controlled trials were included, in which 147 older adults received HMB intervention and 140 were assigned to control groups. The meta-analysis showed greater muscle mass gain in the intervention groups compared with the control groups (standard mean difference=0.352kg; 95% confidence interval: 0.11, 0.594; Z value=2.85; P=0.004). There were no significant fat mass changes between intervention and control groups (standard mean difference=-0.08kg; 95% confidence interval: -0.32, 0.159; Z value=0.66; P=0.511).
CONCLUSION: Beta-hydroxy-beta-methylbutyrate supplementation contributed to preservation of muscle mass in older adults. HMB supplementation may be useful in the prevention of muscle atrophy induced by bed rest or other factors. Further studies are needed to determine the precise effects of HMB on muscle strength and physical function in older adults. - ^ a b c d e f Argilés JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Mañas L (June 2016). "Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease". J. Am. Med. Dir. Assoc. doi:10.1016/j.jamda.2016.04.019. PMID 27324808.
Studies suggest dietary protein and leucine or its metabolite b-hydroxy b-methylbutyrate (HMB) can improve muscle function, in turn improving functional performance. ... These have identified the leucine metabolite β-hydroxy β-methylbutyrate (HMB) as a potent stimulator of protein synthesis as well as an inhibitor of protein breakdown in the extreme case of cachexia.65, 72, 76, 77, 78, 79, 80, 81, 82, 83, 84 A growing body of evidence suggests HMB may help slow, or even reverse, the muscle loss experienced in sarcopenia and improve measures of muscle strength.44, 65, 72, 76, 77, 78, 79, 80, 81, 82, 83, 84 However, dietary leucine does not provide a large amount of HMB: only a small portion, as little as 5%, of catabolized leucine is metabolized into HMB.85 Thus, although dietary leucine itself can lead to a modest stimulation of protein synthesis by producing a small amount of HMB, direct ingestion of HMB more potently affects such signaling, resulting in demonstrable muscle mass accretion.71, 80 Indeed, a vast number of studies have found that supplementation of HMB to the diet may reverse some of the muscle loss seen in sarcopenia and in hypercatabolic disease.65, 72, 83, 86, 87 The overall treatment of muscle atrophy should include dietary supplementation with HMB, although the optimal dosage for each condition is still under investigation.68 ...
Figure 4: Treatments for sarcopenia. It is currently recommended that patients at risk of or suffering from sarcopenia consume a diet high in protein, engage in resistance exercise, and take supplements of the leucine metabolite HMB.{{cite journal}}: External link in|quote= - ^ Portal S, Eliakim A, Nemet D, Halevy O, Zadik Z (July 2010). "Effect of HMB supplementation on body composition, fitness, hormonal profile and muscle damage indices". J. Pediatr. Endocrinol. Metab. 23 (7): 641–650. doi:10.1515/jpem.2010.23.7.641. PMID 20857835.
- ^ a b c d e f Luckose F, Pandey MC, Radhakrishna K (2015). "Effects of amino acid derivatives on physical, mental, and physiological activities". Crit. Rev. Food Sci. Nutr. 55 (13): 1793–1807. doi:10.1080/10408398.2012.708368. PMID 24279396.
HMB, a derivative of leucine, prevents muscle damage and increases muscle strength by reducing exercise-induced proteolysis in muscles and also helps in increasing lean body mass. ... HMB is converted to HMB-CoA which is then used for the synthesis of cholesterol in muscle cells (Nissen and Abumrad, 1997). Cholesterol is needed for the growth, repair, and stabilization of cellular membranes during exercise (Chen, 1984). ... The meta analysis studies and the individual studies conducted support the use of HMB as an effective aid to increase body strength, body composition, and to prevent muscle damage during resistance training.
- ^ a b c d e f g h Molfino A, Gioia G, Rossi Fanelli F, Muscaritoli M (December 2013). "Beta-hydroxy-beta-methylbutyrate supplementation in health and disease: a systematic review of randomized trials". Amino Acids. 45 (6): 1273–1292. doi:10.1007/s00726-013-1592-z. PMID 24057808.
Normally, an individual metabolizes 60 g of L-LEU to obtain 3 g of HMB but a 70 kg person produces 0.2–0.4 g of HMB per day, depending on the dose of LEU in the diet (Van Koevering and Nissen 1992).
- ^ a b c d Ehling S, Reddy TM (September 2015). "Direct Analysis of Leucine and Its Metabolites β-Hydroxy-β-methylbutyric Acid, α-Ketoisocaproic Acid, and α-Hydroxyisocaproic Acid in Human Breast Milk by Liquid Chromatography-Mass Spectrometry". J. Agric. Food Chem. 63 (34): 7567–7573. doi:10.1021/acs.jafc.5b02563. PMID 26271627.
- ^ a b Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, D'Angelo E, Sisto A, Marzetti E (May 2016). "Protein Intake and Muscle Health in Old Age: From Biological Plausibility to Clinical Evidence". Nutrients. 8 (5): 295. doi:10.3390/nu8050295. PMC 4882708. PMID 27187465.
Given the role of leucine as the master dietary regulator of muscle protein turnover, the ingestion of protein sources enriched with this essential amino acid, or its metabolite β-hydroxy β-methylbutyrate, is thought to offer the greatest benefit in terms of preservation of muscle mass and function in old age. ... Recently, it has been demonstrated that β-hydroxy β-methylbutyrate (HMB)—an amino acid metabolite of leucine—is able to stimulate protein synthesis and improve muscle strength and body composition in older adults [67]. ...
6.2. Nutritional Supplementation with HMB
HMB is an active leucine metabolite which activates the mTOR signaling pathway in muscle. Following its absorption, dietary leucine is converted into α-ketoisocaproate (KIC), which is further metabolized into either isovaleryl-CoA or HMB. Under normal conditions, the majority of KIC is converted into isovaleryl-CoA, while only approximately 5% of leucine is metabolized to HMB. This implies that, in order to reach pharmacological levels of HMB, this compound needs to be administered directly, rather than via increasing leucine dosage. It has recently been suggested that HMB may be used to protect or restore muscle mass in older persons with reduced lean body mass [74].
HMB exerts its effects through protective, anticatabolic mechanisms and directly influences protein synthesis. HMB has also been shown to stabilize the muscle cell membrane, to modulate protein degradation and to up-regulate protein synthesis [68]. The daily administration of a nutritional mixture including HMB (2 g), arginine (5 g), and lysine (1.5 g) for 12 weeks was shown to improve physical performance, muscle strength, fat-free mass and protein synthesis in sedentary older women [74]. More recently, Deutz and colleagues [13]—in a multicenter, randomized, placebo-controlled, double-blind trial—demonstrated that the early administration (within 72 h of hospitalization) of a nutrient-dense oral nutritional supplement containing high concentrations of protein and HMB was associated with decreased post-discharge mortality and improved nutritional status in malnourished older adults [13].{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d e Fuller JC, Sharp RL, Angus HF, Khoo PY, Rathmacher JA (November 2015). "Comparison of availability and plasma clearance rates of β-hydroxy-β-methylbutyrate delivery in the free acid and calcium salt forms". Br. J. Nutr. 114 (9): 1403–1409. doi:10.1017/S0007114515003050. PMID 26373270.
Recently, the free acid form of HMB (HMB-FA) has become commercially available in capsule form (gelcap). The current study was conducted to compare the bioavailability of HMB using the two commercially available capsule forms of HMB-FA and Ca-HMB. ... In conclusion, HMB-FA in capsule form improves clearance rate and availability of HMB compared with Ca-HMB in capsule form.
- ^ a b c Linn J (13 May 2013). "Proteins in Human Health and Performance". Iowa State University. Retrieved 31 July 2016.
Dr. Nissen and his collaborator Dr. Naji N. Abumrad, Professor and Chair, Department of Surgery, Vanderbilt University, discovered beta-hydroxy-beta-methylbutyrate (HMB) and its beneficial effects on human health and performance. HMB is currently marketed nationally by Abbott Laboratories as Revigor™, which is a component of Ensure® Muscle Health, and Juven®, which is a nutritional beverage that is clinically shown to promote healing after injury or surgery.
- ^ a b Wilson GJ, Wilson JM, Manninen AH (2008). "Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: A review". Nutrition & Metabolism. 5: 1. doi:10.1186/1743-7075-5-1. PMC 2245953. PMID 18173841.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d Ehling S, Reddy TM (February 2014). "Investigation of the presence of β-hydroxy-β-methylbutyric acid and α-hydroxyisocaproic acid in bovine whole milk and fermented dairy products by a validated liquid chromatography-mass spectrometry method". J. Agric. Food Chem. 62 (7): 1506–1511. doi:10.1021/jf500026s. PMID 24495238.
- ^ a b c d e f g h i j k Szcześniak KA, Ostaszewski P, Fuller JC, Ciecierska A, Sadkowski T (June 2015). "Dietary supplementation of β-hydroxy-β-methylbutyrate in animals – a review". J Anim Physiol Anim Nutr (Berl). 99 (3): 405–417. doi:10.1111/jpn.12234. PMID 25099672. Retrieved 1 June 2016.
Cholesterol is a major component of the cell membrane, and sarcolemma is the one that relies mainly on de novo synthesis of cholesterol. This is important under stressful conditions when muscle cells may lack the capacity to produce adequate amounts of the cholesterol that is essential to proper functioning of cell membranes. Many biochemical studies have shown that HMB may be a precursor of cholesterol synthesis (Bachhawat et al., 1955; Bloch et al., 1954; Coon et al., 1955; Adamson and Greenberg, 1955; Gey et al., 1957). According to pertinent literature, HMB carbon is incorporated into cholesterol. Therefore, increased intramuscular HMB concentrations may provide readily available substrate for the cholesterol synthesis that is needed to form and stabilize the sarcolemma. ... It is known that HMB supplementation decreases post-exercise levels of enzymes, indicating muscle damage, such as creatinine phosphokinase (CK) and lactate dehydrogenase (LDH), which suggests an enhancement of the muscle cell membrane function. This was shown in numerous studies in humans undergoing both resistance and endurance training (Wilson et al., 2013) ... In theory, HMB use as a precursor to cholesterol could aid in stabilizing muscle cell membranes; however, this has not been confirmed by research studies. The effect of HMB on protein metabolism may in fact help stabilize muscle structure more than any effect HMB may have on cholesterol metabolism in the cell.
- ^ Fitzgerald M (15 May 2014). Diet Cults: The Surprising Fallacy at the Core of Nutrition Fads and a Guide to Healthy Eating for the Rest of Us. Pegasus Books. p. 148. ISBN 9781605985954. Retrieved 31 July 2016.
HMB was discovered in the mid-1990s by Steve Nissen, a researcher at Iowa State University
- ^ a b Rippe JM (2013). "Beta-Hydroxy beta-methylbutyrate". Lifestyle Medicine (2nd ed.). CRC Press. p. 724. ISBN 9781439845448. Retrieved 15 August 2016.
- ^ a b "List of Prohibited Substances and Methods: By Substance". World Anti-Doping Agency. 2016. Retrieved 15 August 2016.
- ^ a b The NCAA Research Staff (January 2006). "NCAA Study of Substance Use Habits of College Student-Athletes" (PDF). National Collegiate Athletic Association. p. 7. Retrieved 24 June 2016.
- ^ a b c d Brook MS, Wilkinson DJ, Phillips BE, Perez-Schindler J, Philp A, Smith K, Atherton PJ (January 2016). "Skeletal muscle homeostasis and plasticity in youth and ageing: impact of nutrition and exercise". Acta. Physiol. (Oxf.). 216 (1): 15–41. doi:10.1111/apha.12532. PMC 4843955. PMID 26010896.
- ^ Mullin GE (February 2014). "Nutrition supplements for athletes: potential application to malnutrition". Nutr. Clin. Pract. 29 (1): 146–147. doi:10.1177/0884533613516130. PMID 24336486.
There are a number of nutrition products on the market that are touted to improve sports performance. HMB appears to be the most promising and to have clinical applications to improve muscle mass and function. Continued research using this nutraceutical to prevent and/or improve malnutrition in the setting of muscle wasting is warranted.
- ^ a b Phillips SM (May 2014). "A brief review of critical processes in exercise-induced muscular hypertrophy". Sports Med. 44 Suppl 1: S71–S77. doi:10.1007/s40279-014-0152-3. PMC 4008813. PMID 24791918.
- ^ a b c d e f g h i j k l Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, Etheridge T, Rathmacher JA, Smith K, Szewczyk NJ, Atherton PJ (June 2013). "Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism" (PDF). J. Physiol. (Lond.). 591 (11): 2911–2923. doi:10.1113/jphysiol.2013.253203. PMC 3690694. PMID 23551944. Retrieved 27 May 2016.
Nonetheless, as the overall MPS response was similar, this cellular signalling distinction did not translate into statistically distinguishable anabolic effects in our primary outcome measure of MPS. ... Interestingly, although orally supplied HMB produced no increase in plasma insulin, it caused a depression in MPB (−57%). Normally, postprandial decreases in MPB (of ∼50%) are attributed to the nitrogen-sparing effects of insulin since clamping insulin at post-absorptive concentrations (5 μU ml−1) while continuously infusing AAs (18 g h−1) did not suppress MPB (Greenhaff et al. 2008), which is why we chose not to measure MPB in the Leu group, due to an anticipated hyperinsulinaemia (Fig. 3C). Thus, HMB reduces MPB in a fashion similar to, but independent of, insulin. These findings are in-line with reports of the anti-catabolic effects of HMB suppressing MPB in pre-clinical models, via attenuating proteasomal-mediated proteolysis in response to LPS (Eley et al. 2008).
- ^ a b c Phillips SM (July 2015). "Nutritional supplements in support of resistance exercise to counter age-related sarcopenia". Adv. Nutr. 6 (4): 452–460. doi:10.3945/an.115.008367. PMC 4496741. PMID 26178029.
- ^ a b c Kornasio R, Riederer I, Butler-Browne G, Mouly V, Uni Z, Halevy O (May 2009). "Beta-hydroxy-beta-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways". Biochim. Biophys. Acta. 1793 (5): 755–763. doi:10.1016/j.bbamcr.2008.12.017. PMID 19211028.
- ^ a b c d e f g h i j k l m n o p Kohlmeier M (2015). "Leucine". Nutrient Metabolism: Structures, Functions, and Genes (2nd ed.). Academic Press. pp. 385–388. ISBN 9780123877840. Retrieved 6 June 2016.
Energy fuel: Eventually, most Leu is broken down, providing about 6.0kcal/g. About 60% of ingested Leu is oxidized within a few hours ... Ketogenesis: A significant proportion (40% of an ingested dose) is converted into acetyl-CoA and thereby contributes to the synthesis of ketones, steroids, fatty acids, and other compounds ...
Figure 8.57: Metabolism of L-leucine{{cite book}}: External link in|quote= - ^ "KEGG Reaction: R04137". Kyoto Encyclopedia of Genes and Genomes. Kanehisa Laboratories. Retrieved 24 June 2016.
- ^ a b "KEGG Reaction: R10759". Kyoto Encyclopedia of Genes and Genomes. Kanehisa Laboratories. Retrieved 24 June 2016.
- ^ a b Mock DM, Stratton SL, Horvath TD, Bogusiewicz A, Matthews NI, Henrich CL, Dawson AM, Spencer HJ, Owen SN, Boysen G, Moran JH (November 2011). "Urinary excretion of 3-hydroxyisovaleric acid and 3-hydroxyisovaleryl carnitine increases in response to a leucine challenge in marginally biotin-deficient humans". J. Nutr. 141 (11): 1925–1930. doi:10.3945/jn.111.146126. PMC 3192457. PMID 21918059.
Reduced activity of MCC impairs catalysis of an essential step in the mitochondrial catabolism of the BCAA leucine. Metabolic impairment diverts methylcrotonyl CoA to 3-hydroxyisovaleryl CoA in a reaction catalyzed by enoyl-CoA hydratase (22, 23). 3-Hydroxyisovaleryl CoA accumulation can inhibit cellular respiration either directly or via effects on the ratios of acyl CoA:free CoA if further metabolism and detoxification of 3-hydroxyisovaleryl CoA does not occur (22). The transfer to carnitine by 4 carnitine acyl-CoA transferases distributed in subcellular compartments likely serves as an important reservoir for acyl moieties (39–41). 3-Hydroxyisovaleryl CoA is likely detoxified by carnitine acetyltransferase producing 3HIA-carnitine, which is transported across the inner mitochondrial membrane (and hence effectively out of the mitochondria) via carnitine-acylcarnitine translocase (39). 3HIA-carnitine is thought to be either directly deacylated by a hydrolase to 3HIA or to undergo a second CoA exchange to again form 3-hydroxyisovaleryl CoA followed by release of 3HIA and free CoA by a thioesterase.
- ^ "Homo sapiens: 4-hydroxyphenylpyruvate dioxygenase reaction". MetaCyc. SRI International. 20 August 2012. Retrieved 6 June 2016.
- ^ a b "Leucine metabolism". BRENDA. Technische Universität Braunschweig. Retrieved 12 August 2016.
- ^ a b c d e f g h i j k l m n o p q r s "3-Hydroxyisovaleric acid". HMDB Version 3.6. Human Metabolome Database. 11 February 2016. Retrieved 25 May 2016.
- ^ a b c "3-hydroxyisovaleric acid". Chemical Entities of Biological Interest. European Bioinformatics Institute. 23 October 2015. Retrieved 20 August 2016.
- ^ "3-hydroxyisovalerate". Chemical Entities of Biological Interest. European Bioinformatics Institute. 16 September 2014. Retrieved 20 August 2016.
- ^ WO application 2015094925, White TO, "Stable liquid filled hard capsule comprising beta-hydroxy-beta-methylbutyric acid", published 25 June 2015, assigned to Capsugel Belgium Nv
- ^ "Beta-Hydroxyisovaleric acid". ChemicalBook. Retrieved 20 August 2016.
- ^ Coffman DD, Cramer R, Mochel WE (June 1958). "Syntheses by Free-radical Reactions. V. A New Synthesis of Carboxylic Acids". Journal of the American Chemical Society. 80 (11): 2882–2887. doi:10.1021/ja01544a072.
- ^ Doraiswamy LK (2001). "Example 5.2". Organic Synthesis Engineering. New York: Oxford University Press. pp. 102–4. ISBN 978-0-19-509689-7.
- ^ Lee IY, Nissen SL, Rosazza JP (1997). "Conversion of beta-methylbutyric acid to beta-hydroxy-beta-methylbutyric acid by Galactomyces reessii" (PDF). Applied and Environmental Microbiology. 63 (11): 4191–5. PMC 168736. PMID 9361403.
External links
- Beta-Hydroxyisovaleric acid at the U.S. National Library of Medicine Medical Subject Headings (MeSH)