Gut–brain axis

| Part of a series on |
| Microbiomes |
|---|
 |
The gut–brain axis is the two-way biochemical signaling that takes place between the gastrointestinal tract (GI tract) and the central nervous system (CNS).[2] The term "microbiota–gut–brain axis" highlights the role of gut microbiota in these biochemical signaling .[3][2] Broadly defined, the gut–brain axis includes the central nervous system, neuroendocrine system, neuroimmune systems, the hypothalamic–pituitary–adrenal axis (HPA axis), sympathetic and parasympathetic arms of the autonomic nervous system, the enteric nervous system, vagus nerve, and the gut microbiota.[2]
Chemicals released by the gut microbiome can influence brain development, starting from birth. A review from 2015 states that the gut microbiome influences the CNS by "regulating brain chemistry and influencing neuro-endocrine systems associated with stress response, anxiety and memory function".[4] The gut, sometimes referred to as the "second brain", may use the same type of neural network as the CNS, suggesting why it could have a role in brain function and mental health.[5]
The bidirectional communication is done by immune, endocrine, humoral and neural connections between the gastrointestinal tract and the central nervous system.[4] More research suggests that the gut microbiome influence the function of the brain by releasing the following chemicals: cytokines, neurotransmitters, neuropeptides, chemokines, endocrine messengers and microbial metabolites such as "short-chain fatty acids, branched chain amino acids, and peptidoglycans".[6] These chemical signals are then transported to the brain via the blood, neuropod cells, nerves, endocrine cells,[7][8] where they impact different metabolic processes. Studies have confirmed that gut microbiome contribute to range of brain functions controlled by the hippocampus, prefrontal cortex and amygdala (responsible for emotions and motivation) and act as a key node in the gut-brain behavioral axis.[9]
While Irritable bowel syndrome (IBS) is the only disease confirmed to be directly influenced by the gut microbiome, many disorders (such as anxiety, autism, depression and schizophrenia) have been reportedly linked to the gut-brain axis as well.[6][10][7] According to a study from 2017, "probiotics have the ability to restore normal microbial balance, and therefore have a potential role in the treatment and prevention of anxiety and depression".[11]
The first of the brain–gut interactions shown, was the cephalic phase of digestion, in the release of gastric and pancreatic secretions in response to sensory signals, such as the smell and sight of food. This was first demonstrated by Pavlov through Nobel prize winning research in 1904.[12][13]
As of October 2016, most of the work done on the role of gut microbiota in the gut–brain axis had been conducted in animals, or on characterizing the various neuroactive compounds that gut microbiota can produce. Studies with humans – measuring variations in gut microbiota between people with various psychiatric and neurological conditions or when stressed, or measuring effects of various probiotics (dubbed "psychobiotics" in this context) – had generally been small and were just beginning to be generalized.[14] Whether changes to the gut microbiota are a result of disease, a cause of disease, or both in any number of possible feedback loops in the gut–brain axis, remain unclear.[15]
Enteric nervous system
[edit]
The enteric nervous system is one of the main divisions of the nervous system and consists of a mesh-like system of neurons that governs the function of the gastrointestinal system; it has been described as a "second brain" for several reasons. The enteric nervous system can operate autonomously. It normally communicates with the central nervous system (CNS) through the parasympathetic (e.g., via the vagus nerve) and sympathetic (e.g., via the prevertebral ganglia) nervous systems. However, vertebrate studies show that when the vagus nerve is severed, the enteric nervous system continues to function.[16]
In vertebrates, the enteric nervous system includes efferent neurons, afferent neurons, and interneurons, all of which make the enteric nervous system capable of carrying reflexes in the absence of CNS input. The sensory neurons report on mechanical and chemical conditions. Through intestinal muscles, the motor neurons control peristalsis and churning of intestinal contents. Other neurons control the secretion of enzymes. The enteric nervous system also makes use of more than 30 neurotransmitters, most of which are identical to the ones found in CNS, such as acetylcholine, dopamine, and serotonin. More than 90% of the body's serotonin lies in the gut, as well as about 50% of the body's dopamine; the dual function of these neurotransmitters is an active part of gut–brain research.[17][18][19]
The first of the gut–brain interactions was shown to be between the sight and smell of food and the release of gastric secretions, known as the cephalic phase, or cephalic response of digestion.[12][13]
Tryptophan metabolism by human gut microbiota ()
|
Gut microbiota
[edit]

The gut microbiota is the complex community of microorganisms that live in the digestive tracts of humans and other animals. The gut metagenome is the aggregate of all the genomes of gut microbiota.[24] The gut is one niche that human microbiota inhabit.[25]
In humans, the gut microbiota has the largest quantity of bacteria and the greatest number of species, compared to other areas of the body.[26] In humans, the gut flora is established at one to two years after birth; by that time, the intestinal epithelium and the intestinal mucosal barrier that it secretes have co-developed in a way that is tolerant to, and even supportive of, the gut flora and that also provides a barrier to pathogenic organisms.[27][28]
The relationship between gut microbiota and humans is not merely commensal (a non-harmful coexistence), but rather a mutualistic relationship.[25] Human gut microorganisms benefit the host by collecting the energy from the fermentation of undigested carbohydrates and the subsequent absorption of short-chain fatty acids (SCFAs), acetate, butyrate, and propionate.[26][29] Intestinal bacteria also play a role in synthesizing vitamin B and vitamin K as well as metabolizing bile acids, sterols, and xenobiotics.[25][29] The systemic importance of the SCFAs and other compounds they produce are like hormones and the gut flora itself appears to function like an endocrine organ;[29] dysregulation of the gut flora has been correlated with a host of inflammatory and autoimmune conditions.[26][30]
The composition of human gut microbiota changes over time, when the diet changes, and as overall health changes.[26][30] In general, the average human has over 1000 species of bacteria in their gut microbiome, with Bacteroidetes and Firmicutes being the dominant phyla. Diets higher in processed foods and unnatural chemicals can negatively alter the ratios of these species, while diets high in whole foods can positively alter the ratios. Additional health factors that may skew the composition of the gut microbiota are antibiotics and probiotics. Antibiotics have severe impacts on gut microbiota, ridding of both good and bad bacteria. Without proper rehabilitation, it can be easy for harmful bacteria to become dominant. Probiotics may help to mitigate this by supplying healthy bacteria into the gut and replenishing the richness and diversity of the gut microbiota. There are many strains of probiotics that can be administered depending on the needs of a specific individual.[31]
Gut–brain integration
[edit]The gut–brain axis, a bidirectional neurohumoral communication system, is important for maintaining homeostasis and is regulated through the central and enteric nervous systems and the neural, endocrine, immune, and metabolic pathways, and especially including the hypothalamic–pituitary–adrenal axis (HPA axis).[2] That term has been expanded to include the role of the gut microbiota as part of the "microbiome-gut-brain axis", a linkage of functions including the gut microbiota.[2]
Interest in the field was sparked by a 2004 study (Nobuyuki Sudo and Yoichi Chida) showing that germ-free mice (genetically homogeneous laboratory mice, birthed and raised in an antiseptic environment) showed an exaggerated HPA axis response to stress, compared to non-GF laboratory mice.[2]
The gut microbiota can produce a range of neuroactive molecules, such as acetylcholine, catecholamines, γ-aminobutyric acid, histamine, melatonin, and serotonin, which are essential for regulating peristalsis and sensation in the gut.[32] Changes in the composition of the gut microbiota due to diet, drugs, or disease correlate with changes in levels of circulating cytokines, some of which can affect brain function.[32] The gut microbiota also release molecules that can directly activate the vagus nerve, which transmits information about the state of the intestines to the brain.[32]
Likewise, chronic or acutely stressful situations activate the hypothalamic–pituitary–adrenal axis, causing changes in the gut microbiota and intestinal epithelium, and possibly having systemic effects.[32] Additionally, the cholinergic anti-inflammatory pathway, signaling through the vagus nerve, affects the gut epithelium and microbiota.[32] Hunger and satiety are integrated in the brain, and the presence or absence of food in the gut and types of food present also affect the composition and activity of gut microbiota.[32]
Most of the work that has been done on the role of gut microbiota in the gut–brain axis has been conducted in animals, including the highly artificial germ-free mice. As of 2016, studies with humans measuring changes to gut microbiota in response to stress, or measuring effects of various probiotics, have generally been small and cannot be generalized; whether changes to gut microbiota are a result of disease, a cause of disease, or both in any number of possible feedback loops in the gut–brain axis, remains unclear.[15]
The concept is of special interest in autoimmune diseases such as multiple sclerosis.[33] This process is thought to be regulated via the gut microbiota, which ferment indigestible dietary fibre and resistant starch; the fermentation process produces short chain fatty acids (SCFAs) such as propionate, butyrate, and acetate.[34] The history of ideas about a relationship between the gut and the mind dates from the nineteenth century. [35]
Clinical significance
[edit]While Irritable bowel syndrome (IBS) is the only disease confirmed to be directly influenced by the gut microbiome, many disorders such as anxiety, autism, depression and schizophrenia have been linked to the gut-brain axis as well.[6][36][7]
Skin conditions
[edit]Skin conditions such as acne were proposed as early as 1930,[37] to be related to emotional states which altered the gut microbiome leading to systemic inflammation. Such conditions that have been improved by the use of probiotics.[38] Studies have shown overlapping mechanisms in psoriasis and depression; psoriasis causing disturbances in the gut microbiota that reflect in the brain causing depression that in turn can cause the stress that affects the microbiome.[39] Probiotics may reduce symptoms of depression through the vagus nerve and sympathetic pathways.
Irritable bowel syndrome
[edit]Irritable bowel syndrome (IBS) can cause many abdominal issues such as symptoms of constipation, diarrhea, gas, bloating, and abdominal pain. IBS can be stress-induced and flare-ups are associated with bouts of stress. The gut-brain axis may explain this. The use of probiotics has been shown to help to restore a balance of helpful and harmful bacteria.[40]
Anxiety
[edit]Brain function is dependent on multiple neuropeptides including dopamine, GABA and serotonin, that are controlled in the gut microbiota. Imbalances in the gut microbiota intensifies anxiety as both the immune and metabolic pathways are affected. Specific microbes can lead to increased anxiety due to the activation of c-Fos proteins. These proteins serve as indicators of neuronal activation. Probiotics have beneficial impacts on anxiety.[41]
Autism
[edit]Studies have shown that children with autism are four times more likely to develop gastrointestinal disorders. The severity of their behavioral symptoms is proportional to the severity of their gastrointestinal issues. Many children with autism have high focal levels of HMGB1.[42] [43]
Schizophrenia
[edit]Different neurotrophins play a role in schizophrenia. One of the main ones is called Brain-Derived Neurotrophic Factor (BDNF). BDNF has been associated with schizophrenia and is believed to be a part of the molecular mechanism that has to do with cognitive dysfunction during neurodevelopmental changes. Those who have been diagnosed with schizophrenia tend to exhibit lower levels of BDNF in blood and levels of BDNF are also lower in the cortex and hippocampus. Levels of butyric acid have also been shown to be different between schizophrenic patients and non-schizophrenic patients. It is important to note that studies regarding the link between the gut-brain axis and schizophrenia are limited and further studies are underway. [44]
Parkinson's disease
[edit]Braak's theory proposed that gut dysbiosis in Parkinson's causes the aggregation of alpha-synuclein in the gastrointestinal tract before its spreading to the brain.[45]
Bile acids and cognitive function
[edit]Microbial derived secondary bile acids produced in the gut may influence cognitive function.[46] Altered bile acid profiles occur in cases of mild cognitive impairment and Alzheimer's disease with an increase in cytotoxic secondary bile acids and a decrease in primary bile acids.[47] These findings suggest a role of the gut microbiome in the progression to Alzheimer's disease.[47] In contrast to the cytotoxic effect of secondary bile acids, the bile acid tauroursodeoxycholic acid may be beneficial in the treatment of neurodegenerative diseases.[48]
As more bile acids are absorbed via apical sodium-bile acid transporters, there is a significant increase in age-related cognitive impairment. Levels of serum conjugated primary bile acids were monitored and increased levels revealed ammonia accumulation in the brain. These increased levels of ammonia led to hippocampal synapse loss. Because the hippocampus is largely responsible for memory, the loss of these synapses can have profound impacts on the memories of those affected. [49]
References
[edit]- ^ Chao, Yin-Xia; Gulam, Muhammad Yaaseen; Chia, Nicholas Shyh Jenn; Feng, Lei; Rotzschke, Olaf; Tan, Eng-King (2020). "Gut–Brain Axis: Potential Factors Involved in the Pathogenesis of Parkinson's Disease". Frontiers in Neurology. 11: 849. doi:10.3389/fneur.2020.00849. ISSN 1664-2295. PMC 7477379. PMID 32982910.
- ^ a b c d e f Mayer, EA; Knight, R; Mazmanian, SK; et al. (2014). "Gut microbes and the brain: paradigm shift in neuroscience". The Journal of Neuroscience. 34 (46): 15490–15496. doi:10.1523/JNEUROSCI.3299-14.2014. PMC 4228144. PMID 25392516.
- ^ Wang, Y; Kasper, LH (May 2014). "The role of microbiome in central nervous system disorders". Brain, Behavior, and Immunity. 38: 1–12. doi:10.1016/j.bbi.2013.12.015. PMC 4062078. PMID 24370461.
- ^ a b Carabotti, Marilia (2015). "The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems". Annals of Gastroenterology. 28 (2): 203–209. PMC 4367209. PMID 25830558.
- ^ "Gut-Brain Connection: What It is, Behavioral Treatments". Cleveland Clinic. Retrieved 2022-06-01.
- ^ a b c Cryan, John F; O'Riordan, Kenneth J; Cowan, Caitlin; Kiran, Sandhu; Bastiaanssen, Thomaz; Boehme, Marcus (2019). "The Microbiota-Gut-Brain Axis". Physiological Reviews. 99 (4): 1877–2013. doi:10.1152/physrev.00018.2018. hdl:10468/10506. PMID 31460832. S2CID 201661076.
- ^ a b c Chen, Yijing; Xu, Jinying; Chen, Yu (13 June 2021). "Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders". Nutrients. 13 (6): 2099. doi:10.3390/nu13062099. PMC 8234057. PMID 34205336.
- ^ Kaelberer, Melanie Maya; Rupprecht, Laura E.; Liu, Winston W.; Weng, Peter; Bohórquez, Diego V. (2020-07-08). "Neuropod Cells: The Emerging Biology of Gut-Brain Sensory Transduction". Annual Review of Neuroscience. 43 (1): 337–353. doi:10.1146/annurev-neuro-091619-022657. ISSN 0147-006X. PMC 7573801. PMID 32101483.
- ^ Cowan, Caitlin S M; Hoban, Alan E; Ventura-Silva, Ana Paula; Dinan, Timothy G; Clarke, Gerard; Cryan, John F (17 November 2017). "Gutsy Moves: The Amygdala as a Critical Node in Microbiota to Brain Signaling". BioEssays. 40 (1). doi:10.1002/bies.201700172. hdl:10468/5116. PMID 29148060. S2CID 205478039.
- ^ Dolan, Eric W. (2023-05-19). "New study links disturbed energy metabolism in depressed individuals to disruption of the gut microbiome". PsyPost. Retrieved 2023-05-19.
- ^ Clapp, Megan; Aurora, Nadia; Herrera, Lindsey; Bhatia, Manisha; Wilen, Emily; Wakefield, Sarah (15 September 2017). "Gut Microbiota's Effect on Mental Health: The Gut-Brain Axis". Clinics and Practice. 7 (4): 987. doi:10.4081/cp.2017.987. PMC 5641835. PMID 29071061.
- ^ a b Filaretova, L; Bagaeva, T (2016). "The Realization of the Brain–Gut Interactions with Corticotropin-Releasing Factor and Glucocorticoids". Current Neuropharmacology. 14 (8): 876–881. doi:10.2174/1570159x14666160614094234. PMC 5333583. PMID 27306034.
- ^ a b Smeets, PA; Erkner, A; de Graaf, C (November 2010). "Cephalic phase responses and appetite". Nutrition Reviews. 68 (11): 643–655. doi:10.1111/j.1753-4887.2010.00334.x. PMID 20961295.
- ^ Wang, Huiying; Lee, In-Seon; Braun, Christoph; Enck, Paul (October 2016). "Effect of Probiotics on Central Nervous System Functions in Animals and Humans: A Systematic Review". Journal of Neurogastroenterology and Motility. 22 (4): 589–605. doi:10.5056/jnm16018. PMC 5056568. PMID 27413138.
- ^ a b Schneiderhan, J; Master-Hunter, T; Locke, A (2016). "Targeting gut flora to treat and prevent disease". The Journal of Family Practice. 65 (1): 34–38. PMID 26845162. Archived from the original on 2016-08-15. Retrieved 2016-06-25.
- ^ Li, Ying; Owyang, Chung (September 2003). "Musings on the Wanderer: What's New in Our Understanding of Vago-Vagal Reflexes? V. Remodeling of vagus and enteric neural circuitry after vagal injury". American Journal of Physiology. Gastrointestinal and Liver Physiology. 285 (3): G461–469. doi:10.1152/ajpgi.00119.2003. PMID 12909562.
- ^ Pasricha, Pankaj Jay (2 March 2011). "Stanford Hospital: Brain in the Gut – Your Health". YouTube.
- ^ Martinucci, I; et al. (2015). "Genetics and pharmacogenetics of aminergic transmitter pathways in functional gastrointestinal disorders". Pharmacogenomics. 16 (5): 523–539. doi:10.2217/pgs.15.12. hdl:11577/3166305. PMID 25916523.
- ^ Smitka, K; et al. (2013). "The role of "mixed" orexigenic and anorexigenic signals and autoantibodies reacting with appetite-regulating neuropeptides and peptides of the adipose tissue-gutbrain axis: relevance to food intake and nutritional status in patients with anorexia nervosa and bulimia nervosa". International Journal of Endocrinology. 2013: 483145. doi:10.1155/2013/483145. PMC 3782835. PMID 24106499.
- ^ a b c d e f g h i Zhang LS, Davies SS (April 2016). "Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions". Genome Med. 8 (1): 46. doi:10.1186/s13073-016-0296-x. PMC 4840492. PMID 27102537.
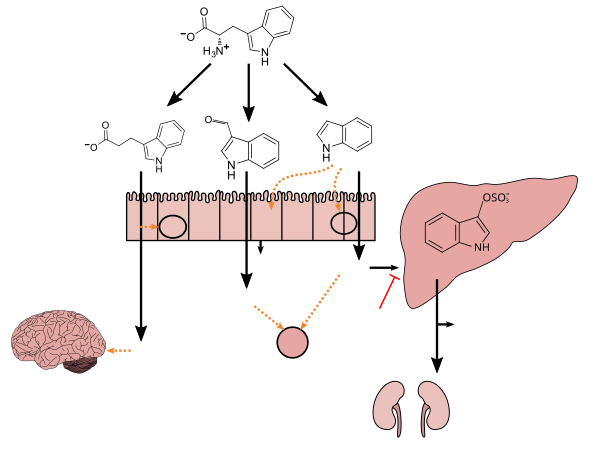
Lactobacillus spp. convert tryptophan to indole-3-aldehyde (I3A) through unidentified enzymes [125]. Clostridium sporogenes convert tryptophan to IPA [6], likely via a tryptophan deaminase. ... IPA also potently scavenges hydroxyl radicals
Table 2: Microbial metabolites: their synthesis, mechanisms of action, and effects on health and disease
Figure 1: Molecular mechanisms of action of indole and its metabolites on host physiology and disease - ^ Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G (March 2009). "Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites". Proc. Natl. Acad. Sci. U.S.A. 106 (10): 3698–3703. Bibcode:2009PNAS..106.3698W. doi:10.1073/pnas.0812874106. PMC 2656143. PMID 19234110.
Production of IPA was shown to be completely dependent on the presence of gut microflora and could be established by colonization with the bacterium Clostridium sporogenes.
IPA metabolism diagram - ^ "3-Indolepropionic acid". Human Metabolome Database. University of Alberta. Retrieved 12 June 2018.
- ^ Chyan YJ, Poeggeler B, Omar RA, Chain DG, Frangione B, Ghiso J, Pappolla MA (July 1999). "Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid". J. Biol. Chem. 274 (31): 21937–21942. doi:10.1074/jbc.274.31.21937. PMID 10419516. S2CID 6630247.
[Indole-3-propionic acid (IPA)] has previously been identified in the plasma and cerebrospinal fluid of humans, but its functions are not known. ... In kinetic competition experiments using free radical-trapping agents, the capacity of IPA to scavenge hydroxyl radicals exceeded that of melatonin, an indoleamine considered to be the most potent naturally occurring scavenger of free radicals. In contrast with other antioxidants, IPA was not converted to reactive intermediates with pro-oxidant activity.
- ^ Saxena, R.; Sharma, V.K (2016). "A Metagenomic Insight Into the Human Microbiome: Its Implications in Health and Disease". In D. Kumar; S. Antonarakis (eds.). Medical and Health Genomics. Elsevier Science. p. 117. doi:10.1016/B978-0-12-420196-5.00009-5. ISBN 978-0-12-799922-7.
- ^ a b c Sherwood, Linda; Willey, Joanne; Woolverton, Christopher (2013). Prescott's Microbiology (9th ed.). New York: McGraw Hill. pp. 713–721. ISBN 978-0-07-340240-6. OCLC 886600661.
- ^ a b c d Quigley, EM (2013). "Gut bacteria in health and disease". Gastroenterology & Hepatology. 9 (9): 560–569. PMC 3983973. PMID 24729765.
- ^ Sommer, F; Bäckhed, F (Apr 2013). "The gut microbiota--masters of host development and physiology". Nature Reviews Microbiology. 11 (4): 227–238. doi:10.1038/nrmicro2974. PMID 23435359. S2CID 22798964.
- ^ Faderl, M; et al. (Apr 2015). "Keeping bugs in check: The mucus layer as a critical component in maintaining intestinal homeostasis". IUBMB Life. 67 (4): 275–285. doi:10.1002/iub.1374. PMID 25914114. S2CID 25878594.
- ^ a b c Clarke, G; et al. (1 August 2014). "Minireview: Gut microbiota: the neglected endocrine organ". Molecular Endocrinology. 28 (8): 1221–1238. doi:10.1210/me.2014-1108. PMC 5414803. PMID 24892638.
- ^ a b Shen, S; Wong, CH (Apr 2016). "Bugging inflammation: role of the gut microbiota". Clinical & Translational Immunology. 5 (4): e72. doi:10.1038/cti.2016.12. PMC 4855262. PMID 27195115.
- ^ Hemarajata, Peera; Versalovic, James (2013). "Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation". Therapeutic Advances in Gastroenterology. 6 (1): 39–51. doi:10.1177/1756283X12459294. ISSN 1756-2848. PMC 3539293. PMID 23320049.
- ^ a b c d e f Petra, AI; et al. (May 2015). "Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders With Suspected Immune Dysregulation". Clinical Therapeutics. 37 (5): 984–995. doi:10.1016/j.clinthera.2015.04.002. PMC 4458706. PMID 26046241.
- ^ Parodi, Benedetta; Kerlero de Rosbo, Nicole (2021-09-21). "The Gut-Brain Axis in Multiple Sclerosis. Is Its Dysfunction a Pathological Trigger or a Consequence of the Disease?". Frontiers in Immunology. 12: 718220. doi:10.3389/fimmu.2021.718220. ISSN 1664-3224. PMC 8490747. PMID 34621267.
- ^ Melbye, Pernille; Olsson, Anna; Hansen, Tue H.; Søndergaard, Helle B.; Bang Oturai, Annette (2019-03-01). "Short-chain fatty acids and gut microbiota in multiple sclerosis". Acta Neurologica Scandinavica. 139 (3): 208–219. doi:10.1111/ane.13045. PMID 30427062.
- ^ Miller, Ian (2018-11-08). "The gut–brain axis: historical reflections". Microbial Ecology in Health and Disease. 29 (2). Informa UK Limited: 1542921. doi:10.1080/16512235.2018.1542921. ISSN 1651-2235. PMC 6225396. PMID 30425612.
- ^ Dolan, Eric W. (2023-05-19). "New study links disturbed energy metabolism in depressed individuals to disruption of the gut microbiome". PsyPost. Retrieved 2023-05-19.
- ^ Stokes; Pillsbury (December 1930). "The effect on the skin of emotional and nervous states: Theoretical and practical consideration of a gastro-intestinal mechanism". Archives of Dermatology and Syphilology. 22 (6): 962–993. doi:10.1001/archderm.1930.01440180008002.
- ^ Bowe, W. P.; Logan, A. C. (2011). "Acne vulgaris, probiotics and the gut-brain-skin axis – back to the future?". Gut Pathogens. 3 (1): 1. doi:10.1186/1757-4749-3-1. PMC 3038963. PMID 21281494.
- ^ Wang, Xiaoxu; Li, Yafan; Wu, Lingjun; Xiao, Shiju; Ji, Yunrun; Tan, Yong; Jiang, Chunyan; Zhang, Guangzhong (2021-05-01). "Dysregulation of the gut-brain-skin axis and key overlapping inflammatory and immune mechanisms of psoriasis and depression". Biomedicine & Pharmacotherapy. 137: 111065. doi:10.1016/j.biopha.2020.111065. ISSN 0753-3322. PMID 33540138.
- ^ Chen, Minjia; Ruan, Guangcong; Chen, Lu; Ying, Senhong; Li, Guanhu; Xu, Fenghua; Xiao, Zhifeng; Tian, Yuting; Lv, Linling; Ping, Yi; Cheng, Yi; Wei, Yanling (2022-02-16). "Neurotransmitter and Intestinal Interactions: Focus on the Microbiota-Gut-Brain Axis in Irritable Bowel Syndrome". Frontiers in Endocrinology. 13. doi:10.3389/fendo.2022.817100. ISSN 1664-2392. PMC 8888441. PMID 35250873.
- ^ Ferrari, Sara; Mulè, Simone; Parini, Francesca; Galla, Rebecca; Ruga, Sara; Rosso, Giorgia; Brovero, Arianna; Molinari, Claudio; Uberti, Francesca (2024-05-01). "The influence of the gut-brain axis on anxiety and depression: A review of the literature on the use of probiotics". Journal of Traditional and Complementary Medicine. 14 (3): 237–255. doi:10.1016/j.jtcme.2024.03.011. ISSN 2225-4110. PMID 38707924.
- ^ Carissimi, Claudia; Laudadio, Ilaria; Palone, Francesca (October 2019). "Functional analysis of gut microbiota and immunoinflammation in children with autism spectrum disorders". Digestive and Liver Disease. 51 (10): 1366–1374. doi:10.1016/j.dld.2019.06.006.
- ^ Gonçalves, Cinara L.; Doifode, Tejaswini; Rezende, Victoria L.; Costa, Maiara A.; Rhoads, J. Marc; Soutullo, Cesar A. (2024-01-15). "The many faces of microbiota-gut-brain axis in autism spectrum disorder". Life Sciences. 337: 122357. doi:10.1016/j.lfs.2023.122357. ISSN 0024-3205. PMID 38123016.
- ^ Nemani, Katlyn; Hosseini Ghomi, Reza; McCormick, Beth; Fan, Xiaoduo (2015-01-02). "Schizophrenia and the gut–brain axis". Progress in Neuro-Psychopharmacology and Biological Psychiatry. 56: 155–160. doi:10.1016/j.pnpbp.2014.08.018. ISSN 0278-5846. PMID 25240858.
- ^ Lubomski, Michal; Tan, Ai Huey; Lim, Shen-Yang; Holmes, Andrew J.; Davis, Ryan L.; Sue, Carolyn M. (2020-09-01). "Parkinson's disease and the gastrointestinal microbiome". Journal of Neurology. 267 (9): 2507–2523. doi:10.1007/s00415-019-09320-1. ISSN 1432-1459. PMID 31041582. S2CID 141624748.
- ^ Connell, Emily; Le Gall, Gwenaelle; Pontifex, Matthew G.; Sami, Saber; Cryan, John F.; Clarke, Gerard; Müller, Michael; Vauzour, David (2022). "Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia". Molecular Neurodegeneration. 17 (1): 43. doi:10.1186/s13024-022-00548-6. PMC 9204954. PMID 35715821.
- ^ a b MahmoudianDehkordi S, Arnold M, Nho K, et al. (2019). "Altered bile acid profile associates with cognitive impairment in Alzheimer's disease-An emerging role for gut microbiome". Alzheimer's & Dementia. 15 (1): 76–92. doi:10.1016/j.jalz.2018.07.217. PMC 6487485. PMID 30337151.
- ^ Khalaf, Kareem; Tornese, Paolo; Cocco, Antoniangela; Albanese, Alberto (2022). "Tauroursodeoxycholic acid: A potential therapeutic tool in neurodegenerative diseases". Translational Neurodegeneration. 11 (1): 33. doi:10.1186/s40035-022-00307-z. PMC 9166453. PMID 35659112.
- ^ Ren, Zhenxing; Zhao, Ling; Zhao, Mingliang; Bao, Tianhao; Chen, Tianlu; Zhao, Aihua; Zheng, Xiaojiao; Gu, Xinru; Sun, Tao; Guo, Yuhuai; Tang, Yajun; Xie, Guoxiang; Jia, Wei (2024-05-21). "Increased intestinal bile acid absorption contributes to age-related cognitive impairment". Cell Reports Medicine. 5 (5): 101543. doi:10.1016/j.xcrm.2024.101543. ISSN 2666-3791. PMC 11148718. PMID 38697101.
External links
[edit] Media related to Gut–brain axis at Wikimedia Commons
Media related to Gut–brain axis at Wikimedia Commons