Benzylisoquinoline alkaloids



The benzylisoquinoline alkaloids are natural products that can be classified as isoquinoline alkaloids and are derived from benzylisoquinoline. They also include the benzyl(tetrahydro)isoquinoline alkaloids.
Occurrence[edit]
Benzylisoquinoline alkaloids are found in several plant families, including the poppy family (Papaveraceae), the annonaceae family, and the laurel family.[1] Well-known representatives are primarily found in poppy plants, specifically those from which opium is derived, as well as in actaea.[2] For instance, reticuline has been isolated from Annona reticulata.[3]
Known representatives[edit]
Over 2500 biologically active derivatives are known from the benzylisoquinoline alkaloids.[4] Based on their structure, the compounds can be divided into numerous subgroups: the aporphines, the phthalideisoquinoline alkaloids, the morphinans, the protoberberine alkaloids, and the pavins.[5]
Among the known individual substances in this group are papaverine. Additional examples of compounds in this group are the benzyltetrahydroisoquinoline alkaloids reticuline and laudanosine.[1]
-
Papaverine.
-
(+)-Reticulin
-
(+)-Laudanosine
Properties[edit]
Papaverine has vasodilator and muscle relaxant properties.[3] Laudanosine acts as a tetanic poison.[1]
Biosynthesis[edit]
The biosynthesis of benzylisoquinoline alkaloids has been intensively studied. It begins with the amino acid tyrosine, which is converted to dopamine by hydroxylation and decarboxylation and to 4-hydroxyphenylacetaldehyde by oxidative deamination, respectively. These two compounds are converted into dopamine by enzyme Norcoclaurine synthase catalyzed condensation reaction to form the benzylisoquinoline backbone.[6]
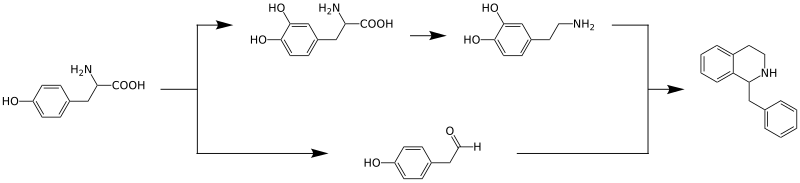
The benzylisoquinoline from this reaction may have different substituents,[6] reticulin is an important intermediate.[5]
References[edit]
- ^ a b c Entry on Benzylisoquinoline alkaloids. at: Römpp Online. Georg Thieme Verlag, retrieved {{{Datum}}}.
- ^ Hermann Hager: Hager's Handbook of Pharmaceutical Practice: Volume 2: Methods, 1133 pages, Springer Publishing (1991), ISBN 978-3-540-52459-5, pp. 35 (Benzylisoquinoline alkaloids, p. 35, at Google Books).
- ^ a b Eberhard Breitmaier (1997), Alkaloide, Wiesbaden: Springer Fachmedien, p. 62, ISBN 9783519035428
- ^ Bettina Ruff: Chemical and Biochemical Methods for the Stereoselective Synthesis of Complex Natural Products, 199 pages, Verlag Logos Berlin (2012), ISBN 978-3-8325-3121-8, pp. 8 (Benzylisoquinoline alkaloids, p. 8, at Google Books).
- ^ a b Jennifer M. Finefield, David H. Sherman, Martin Kreitman, Robert M. Williams: Enantiomeric natural products: occurrence and biogenesis. In Applied Chemistry. 124, 2012, p. 4886-4920, doi:10.1002/ange.201107204.
- ^ a b Yang-Chang Wu (2007), "New Research and Development on the Formosan Annonaceous Plants", Studies in Natural Products Chemistry, Studies in Natural Products Chemistry, vol. 33, pp. 957–1023, doi:10.1016/s1572-5995(06)80044-x, ISBN 9780444527172