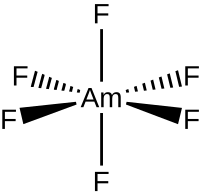
Americium hexafluoride
| |
| Names | |
|---|---|
| Other names
Americium(VI) fluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| AmF6 | |
| Molar mass | 357 g·mol−1 |
| Related compounds | |
Related compounds
|
Uranium hexafluoride Curium hexafluoride Einsteinium hexafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Americium hexafluoride is an inorganic chemical compound of americium metal and fluorine with the chemical formula AmF
6. It is still a hypothetical compound.[1][2] Synthesis by fluorination of americium tetrafluoride was unsuccessfully attempted in 1990.[3] A thermochromatographic identification in 1986 remains inconclusive.[4] Calculations suggest that it may be distorted from octahedral symmetry.[4]
Synthesis[edit]
It is proposed that AmF
6 can be prepared by in both the condensed and gaseous states by the reaction of KrF
2 with AmF
3 in anhydrous HF at 313–333 K.[5]
- AmF3 + KrF2 → AmF6 + Kr[6]
References[edit]
- ^ Meyer, G.; Morss, L. R. (6 December 2012). Synthesis of Lanthanide and Actinide Compounds. Springer Science & Business Media. p. 80. ISBN 978-94-011-3758-4. Retrieved 29 March 2023.
- ^ O'Donnell, T. A. (8 June 2017). The Chemistry of Fluorine: Comprehensive Inorganic Chemistry. Elsevier. p. 1093. ISBN 978-1-4831-4642-3. Retrieved 29 March 2023.
- ^ Malm, J. G.; Weinstock, B.; Weaver, E. E. (1958). "The Preparation and Properties of NpF6; a Comparison with PuF6". The Journal of Physical Chemistry. 62 (12): 1506–1508. doi:10.1021/j150570a009.
- ^ a b Seppelt, Konrad (2015). "Molecular Hexafluorides". Chemical Reviews. 115 (2): 1296–1306. doi:10.1021/cr5001783. PMID 25418862.
- ^ Silva, R. J.; Bidoglio, G.; Robouch, P. B.; Puigdomenech, I.; Wanner, H.; Rand, M. H. (2 December 2012). Chemical Thermodynamics of Americium. Newnes. p. 114. ISBN 978-0-444-59935-3. Retrieved 29 March 2023.
- ^ Прусаков, Владимир Николаевич (2013). Избранные научные труды (in Russian). Rosatom. p. 59. Retrieved 29 March 2023.
