1,8-cineole synthase
| 1,8-cineole synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 4.2.3.108 | ||||||||
| CAS no. | 110637-19-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
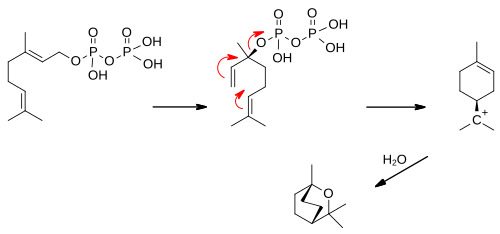
1,8-Cineole synthase (EC 4.2.3.108, 1,8-cineole cyclase, geranyl pyrophoshate:1,8-cineole cyclase, 1,8-cineole synthetase) is an enzyme with systematic name geranyl-diphosphate diphosphate-lyase (cyclizing, 1,8-cineole-forming).[1][2][3][4][5] This enzyme catalyses the following chemical reaction
- geranyl diphosphate + H2O 1,8-cineole + diphosphate
This enzyme requires Mn2+ or Zn2+. Geranyl diphosphate first isomerizes to (S)-linalyl diphosphate which ionises to the alpha-terpinyl cation which reacts with water to form the product.[6]
References[edit]
- ^ Croteau R, Alonso WR, Koepp AE, Johnson MA (February 1994). "Biosynthesis of monoterpenes: partial purification, characterization, and mechanism of action of 1,8-cineole synthase". Archives of Biochemistry and Biophysics. 309 (1): 184–192. doi:10.1006/abbi.1994.1101. PMID 8117108.
- ^ Wise ML, Savage TJ, Katahira E, Croteau R (June 1998). "Monoterpene synthases from common sage (Salvia officinalis). cDNA isolation, characterization, and functional expression of (+)-sabinene synthase, 1,8-cineole synthase, and (+)-bornyl diphosphate synthase". The Journal of Biological Chemistry. 273 (24): 14891–14899. doi:10.1074/jbc.273.24.14891. PMID 9614092.
- ^ Peters RJ, Croteau RB (September 2003). "Alternative termination chemistries utilized by monoterpene cyclases: chimeric analysis of bornyl diphosphate, 1,8-cineole, and sabinene synthases". Archives of Biochemistry and Biophysics. 417 (2): 203–211. doi:10.1016/s0003-9861(03)00347-3. PMID 12941302.
- ^ Chen F, Ro DK, Petri J, Gershenzon J, Bohlmann J, Pichersky E, Tholl D (August 2004). "Characterization of a root-specific Arabidopsis terpene synthase responsible for the formation of the volatile monoterpene 1,8-cineole". Plant Physiology. 135 (4): 1956–1966. doi:10.1104/pp.104.044388. PMC 520767. PMID 15299125.
- ^ Keszei A, Brubaker CL, Carter R, Köllner T, Degenhardt J, Foley WJ (June 2010). "Functional and evolutionary relationships between terpene synthases from Australian Myrtaceae". Phytochemistry. 71 (8–9): 844–852. Bibcode:2010PChem..71..844K. doi:10.1016/j.phytochem.2010.03.013. PMID 20399476.
- ^ Rinkel J, Rabe P, Zur Horst L, Dickschat JS (2016-11-04). "A detailed view on 1,8-cineol biosynthesis by Streptomyces clavuligerus". Beilstein Journal of Organic Chemistry. 12: 2317–2324. doi:10.3762/bjoc.12.225. PMC 5238540. PMID 28144299.
External links[edit]
- 1,8-cineole+synthase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)