Wikipedia talk:WikiProject Chemistry/Archive 22
| This is an archive of past discussions on Wikipedia:WikiProject Chemistry. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 15 | ← | Archive 20 | Archive 21 | Archive 22 | Archive 23 | Archive 24 | Archive 25 |
Chembox
| |||
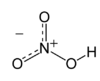
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Nitric acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| 1576 | |||
| KEGG | |||
| MeSH | Nitric+acid | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2031 | ||
| |||
| |||
| Properties | |||
| HNO3 | |||
| Molar mass | 63.012 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Density | 1.5129 g cm-3 | ||
| Melting point | −42 °C (−44 °F; 231 K) | ||
| Boiling point | 83 °C (181 °F; 356 K) | ||
| Completely miscible | |||
| Acidity (pKa) | -1.4 | ||
Refractive index (nD)
|
1.397 (16.5 °C) | ||
| 2.17 ± 0.02 D | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions
|
Nitrous acid | ||
Other cations
|
Sodium nitrate Potassium nitrate Ammonium nitrate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
I am concerned at the size of some of the chemboxes, which means that they stretch far down into the article (example opposite). Would it be possible to arrange for sections, such as Identifiers, Properties etc. to be made into drop-down lists? I have in mind the lists that have a clickable "show" button. It would make the articles much more readable. Perhaps the most important information, such as formula, name(s) could be retained in full view. Petergans (talk) 21:46, 23 January 2011 (UTC)
- Last I knew, multirow chunks of a table could not be collapsed easily. The collapsing of SMILES and InChI works because the collapsible part is full-width, so it looks like it's just a normal row in the table. But it's actually its own complete table or div (see Help:Collapse for details). If the whole Properties section were collapsible, its two-column table layout would be independent of the two-column table layout of the full infobox. As a result, the column-widths would not stay in sync.
| Properties | |||
| melting point | 1 | ||
Indentifiers
| |||
- Setting aside the yucky layout in general, notice that the left-hand label column is not the same size in mp vs SMILES rows. The solution would be to force absolute column-widths, but I think WP recommends avoiding doing that in general. The idea of collapsible sections has been raised a few times at Wikipedia talk:WikiProject Infoboxes (I agree it's a generally-useful idea!) with no solutions:( DMacks (talk) 22:38, 23 January 2011 (UTC)
[1] Chemboxes make right images impossible and readers with a small screen will not be happy with large chemboxes.--Wickey-nl (talk) 11:06, 24 January 2011 (UTC)
- Petergans, problem with only showing the most important information is .. what is 'the most important information'? In a way, that is already possible, one could easily make a /Data subpage, which will automagically appear in the mainpage chembox, and move the original chembox to that /Data subpage, and then make a minimalist page on the top level. But there will still be regular disputes as to what is important and what not.
- I have been experimenting with what DMacks is proposing. It can be made to work, but it does leave uglyness here and there (maybe not big enough to be a serious problem, though). I'll have a second look at that solution.
- Regarding Wickey-nl's remark: small screens are only a problem with the wide chemboxes, which are now largely eliminated (except if you press show on certain InChI's or IUPACNames .. but breaking (e.g. using <br /> marks), especially for InChI's, does break other things or might give problems in the future. The length of a box is not a problem for small screens only, I have a rather big screen here, and I can't fit the whole chembox depicted here on one screen .. I have to scroll anyway. The display of images is indeed an annoying problem.
- Collapsing the sub-boxes is indeed the best solution .. now to get it to work properly. --Dirk Beetstra T C 11:21, 24 January 2011 (UTC)
- Defining the most important information is a right question, indeed. I think, this will be the information the average reader wants to see. That is: images, names and molecular formula. Will always be arbitrary. For chemists, all data are important. Identifiers are secondary important, because they are direct links to many other data (but most likely not interesting for the common reader). Other sections, may be except "Related compounds", are most arbitrary, because they are most specific. Values may be dubious, diverge or hard to verify.--Wickey-nl (talk) 11:47, 24 January 2011 (UTC)
- I agree, name(s), formula and image should always be visible. The rest should be easily accessible. Whether by collasing or link to a separate page is a technical issue for others to work on. Petergans (talk) 11:58, 24 January 2011 (UTC)
- For the last question: needs expanding the box a new request of page data by the browser, like asking for a Data subpage? May be both options can be in the box: button Data subpage? The last will also work if java is not working.--Wickey-nl (talk) 13:18, 24 January 2011 (UTC)
- I agree, name(s), formula and image should always be visible. The rest should be easily accessible. Whether by collasing or link to a separate page is a technical issue for others to work on. Petergans (talk) 11:58, 24 January 2011 (UTC)
- Defining the most important information is a right question, indeed. I think, this will be the information the average reader wants to see. That is: images, names and molecular formula. Will always be arbitrary. For chemists, all data are important. Identifiers are secondary important, because they are direct links to many other data (but most likely not interesting for the common reader). Other sections, may be except "Related compounds", are most arbitrary, because they are most specific. Values may be dubious, diverge or hard to verify.--Wickey-nl (talk) 11:47, 24 January 2011 (UTC)
- Somehow I missed the previous discussion that Wickey-nl mentions. Dirk Beetstra has a good start at User:Beetstra/test. I just hacked on it a bit to solve almost all of the double-bordering ugliness. The collapsible sections do have a slightly thicker border still and mis-matched column-widths. The thicker border actually might not be bad, since it indicates the extent of the collapsible section. DMacks (talk) 16:28, 24 January 2011 (UTC)
- I agree, border is not disturbing.--Wickey-nl (talk) 13:36, 25 January 2011 (UTC)
I created all the sandbox templates. Note 1: this does not work when Java is disabled by the reader. Note 2: Wickey-nl suggested to move Molecular Formula up .. is that really necessary, as then it would be split from the C, H, N-fields (which if moved as well would result in the molweight also showing up there) - moreover, it would not work until all 7000 (?) chemboxes are adapted. What do others think? --Dirk Beetstra T C 19:09, 13 February 2011 (UTC)
I seem to have parameters which break the system. One example is 'OtherCpds' in {{Chembox Related/sandbox}} - add it and the whole does not collapse properly. Similar there is something wrong in {{Chembox Identifiers/sandbox}}. Tinkering and testing needed!! --Dirk Beetstra T C 19:54, 13 February 2011 (UTC)
I seem to have solved the broken parameters .. but would like to see some independent testing in sandboxes first. Thanks. --Dirk Beetstra T C 15:17, 14 February 2011 (UTC)
- I'll be happy to do some testing in my sandbox. What needs to be done to use the provisional chembox? I suggest that we test one collapse at a time rather than leap into a multivariate analysis. How about starting with identifiers? Petergans (talk) 22:56, 17 February 2011 (UTC)
Cossee-Arlman mechanism could use some help
The key mechanistic diagram, File:ZNCosseeArlman.PNG is tagged as being incorrect. One concern is that the mechanism does not support the stereochemistry of Ziegler-Natta polymerizataion. Another is that there are too many electron-pairs moving in the second reaction step. Part of that problem is that the first diagram uses a bond to represent a vacant coordination site and has the CH2 fully bonded to both metals (exceeded octet!) rather than presumably some sort of nonclassical coordination. Would be great if someone could pull out the refs in Cossee-Arlman mechanism or maybe a polymer textbook and straighten it out. DMacks (talk) 20:30, 28 January 2011 (UTC)
- I will look into it. The scheme looks incorrect. Looking at this http://classes.uleth.ca/200701/chem4000a/Class%20Notes%20Web%20Pages/Olefin%20Polymerization.htm, the scheme is possibly over-specific. More tomorrow, I hope.--Smokefoot (talk) 00:54, 29 January 2011 (UTC)
- Which tomorrow? ;-) --Leyo 10:39, 23 February 2011 (UTC)
- I revised the figure a few weeks ago.--Smokefoot (talk) 14:28, 23 February 2011 (UTC)
- Sorry for being blind. I had noticed that there were no edits to the disputed image. --Leyo 16:00, 23 February 2011 (UTC)
- I revised the figure a few weeks ago.--Smokefoot (talk) 14:28, 23 February 2011 (UTC)
- Which tomorrow? ;-) --Leyo 10:39, 23 February 2011 (UTC)
New article: particle
- Cross posted from Wikipedia talk:WikiProject Physics#New article: particle, as this could use an eye from the chemists
Up until recently, we didn't have any article on the concept of a particle, we only had a disambiguation page listing several types of particles, but never a coherent explanation of the concept. It really pissed me off that we lacked a basic explanation of a concept as important as this one, so I got off my ass and fixed that tonight. The "what links here" of particle, particle (disambiguation) and particle physics should probably be cleaned up, so any help doing that would be much appreciated (as well as any expansion of the particle article, of course). Headbomb {talk / contribs / physics / books} 11:53, 8 February 2011 (UTC)
- Thanks for doing this. As a chemist, I thought you did a great job there. Jon C (talk) 17:43, 9 February 2011 (UTC)
Attempt to expand/rewrite physical chemistry
Could those active here have a look at Talk:Physical chemistry? There are some new talk page sections I've started in an attempt to get some advice on how best to rewrite/expand the article. Thanks. Carcharoth (talk) 18:20, 13 February 2011 (UTC)
- I got some useful advice from one editor so far (Smokefoot), and have put up a proposal on the talk page for a new layout - see Talk:Physical chemistry#Proposed layout. It would be great if others have the time to comment as well. Thanks. Carcharoth (talk) 02:07, 16 February 2011 (UTC)
3-D drawings
File:3-aminoisobutyrate.png was recently tagged for speedy because it has {{PD-chem}} as it's permission, and the CSD tag applied stated that A 3D model is not a "Simple structural formula". Does not meet public domain criteria. In the end I removed the CSD and added a {{di-no permission}}, in the hope that the author might update the page. However the author has not logged in since 9 Nov 2009, so that did not happen. Since then User:HJ Mitchell has removed the second template with a summary of not clear enough for speedy. Could well be pd-text or similar. Suggest FfD or PUF. I've no idea how many other structures this might affect, is there some way we can change {{PD-chem}} to explicitly include 3D drawings? - after all on some programs it's not much more effort than a couple of mouse clicks. Ronhjones (Talk) 17:00, 15 February 2011 (UTC)
- With JMol a child can make 3D drawings.--Wickey-nl (talk) 12:15, 16 February 2011 (UTC)
- I do not support the expansion of {{PD-chem}}.
- I have no qualms against removing this specific image -- or all of its close cousins, because they may be eye-candy but is didactically useless. It does not illustrate any important concept; the shape of the molecule is not meaningful (to appl/reactivity); the method of generation is unstated and dubious (expt.? calc.? level of theory? phase?). This relates to Wickey's comment - anyone can make 3D drawing, but it may not make sense to make a 3D drawing at first place.
- We should encourage higher quality 3D depiction (e.g., either with a sound level of theory, or crystallography/NMR) to be CC3.0-by-sa licensed - or if the creator so chooses, dedicate it explicitly to the public domain. Jon C (talk) 19:36, 16 February 2011 (UTC)
Images for deletion
I have nominated a couple of images for deletion at Wikipedia:Files for deletion/2011 February 18. Since probably only chemists will be qualified to address the dispute, I'd like to ask a few chemists to please take a look. Thank you. ChemNerd (talk) 14:24, 18 February 2011 (UTC)
EDIT: Bis(2-ethylhexyl) phthalate - Where to add E.I. mass spectra peak info?
Sorry, didn't realize it had its own talk page. Still learning. Discussion moved to Talk:Bis(2-ethylhexyl)_phthalate.
[DrBurningBunny 20:48, 19 February 2011 (UTC)]
Heck-Matsuda and Soai reaction
The two new article on Heck-Matsuda Reaction and Soai reaction need some help. Both reactions look interesting, but the articles lack a lot. --Stone (talk) 01:37, 2 March 2011 (UTC)
Spark sparks another move request
Spark (fire) → Spark per WP:PRIMARYTOPIC - discussion here. --Kkmurray (talk) 01:56, 2 March 2011 (UTC)
IUPAC new guidelines on atomic weights
The IUPAC has just issued new guidelines for the reporting of atomic weights that should impact the articles on Atomic Weight, the periodic table and the elements H,Li,B,C,N,O,Si,S,Cl and Th. This should be done consistently and with good explanation of the changes.96.250.216.18 (talk) 04:28, 1 March 2011 (UTC)
- I've changed germanium, but would not do anything with other elementboxes. IMHO, this information could be reflected on wikipedia, but this approach is questionable and I would not be surprised if it is rejected/ignored by the science community. Materialscientist (talk) 04:38, 1 March 2011 (UTC)
- I'm not sure our opinions come into it. IUPAC is one of the main standard setting authorities in the community. They don't do these things lightly. Aproposdenada (talk) 05:04, 8 March 2011 (UTC)
Lipscomb
Does anyone else share my concern that User:Jslipscomb is gradually turning Wikipedia into a shrine to his father, William Lipscomb? The latter is undoubtedly an outstanding and well-known scientist, but that doesn't mean that every one of his contributions has to be discussed in great detail. User:Jslipscomb is adding some interesting and well-presented information, but I think it is mostly best kept to the article William Lipscomb. Our article Nuclear magnetic resonance spectroscopy would benefit from a simpler example of an NMR spectrum than File:Lipscomb-NMR-hexaborene-B6H10.png. Some of the recent additions to diborane could be condensed, redirected to Borane#Bonding in boranes, and references to modern sources (preferably textbooks or review articles).
Relevant policies: WP:SECONDARY, Wikipedia:Conflict of interest#Close relationships. WP:OWN.
Ben (talk) 02:27, 7 March 2011 (UTC)
- It's a problem. We need to speak to him about not damaging his Sr Lipscomb's image by self-promotion. --Smokefoot (talk) 02:34, 7 March 2011 (UTC)
Recent changes were made to citations templates (such as {{citation}}, {{cite journal}}, {{cite web}}...). In addition to what was previously supported (bibcode, doi, jstor, isbn, ...), templates now support arXiv, ASIN, JFM, LCCN, MR, OL, OSTI, RFC, SSRN and Zbl. Before, you needed to place |id= (or worse {{arxiv|0123.4567}}|url=http://arxiv.org/abs/0123.4567), now you can simply use |arxiv=0123.4567, likewise for |id= and {{JSTOR|0123456789}}|url=http://www.jstor.org/stable/0123456789 → |jstor=0123456789.
The full list of supported identifiers is given here (with dummy values):
- {{cite journal |author=John Smith |year=2000 |title=How to Put Things into Other Things |journal=Journal of Foobar |volume=1 |issue=2 |pages=3–4 |arxiv=0123456789 |asin=0123456789 |bibcode=0123456789 |doi=0123456789 |jfm=0123456789 |jstor=0123456789 |lccn=0123456789 |isbn=0123456789 |issn=0123456789 |mr=0123456789 |oclc=0123456789 |ol=0123456789 |osti=0123456789 |rfc=0123456789 |pmc=0123456789 |pmid=0123456789 |ssrn=0123456789 |zbl=0123456789 |id={{para|id|____}} }}
Obviously not all citations needs all parameters, but this streamlines the most popular ones and gives both better metadata and better appearances when printed. Headbomb {talk / contribs / physics / books} 02:35, 8 March 2011 (UTC)
Fake elements images
I have nominated several fake elements images for deletion per WP:OR. Please have a look here and there. The commons (2nd) discussion is interesting (it is not about elements, but more about science simulation in general). Materialscientist (talk) 05:30, 20 February 2011 (UTC)
- They have now all been deleted from both en-wiki and commons. Ronhjones (Talk) 20:57, 8 March 2011 (UTC)
Zwitterions
Help! Wickey-nl is teaching himself chemistry by reading the gold book. Can anybody help him understand the difference between a 1,3-dipole and a zwitterion? Chris (talk) 16:51, 11 March 2011 (UTC)
- Others would like to contribute, but first Wickey-nl needs to walk away from this article. When the consensus is clearly against one's views, it is time to stop. Also the tone of the discussions is often unpleasant. You can see that User:Petergans gave up trying to help because minority ideas being pushed so forcefully and so angrily. --Smokefoot (talk) 19:17, 11 March 2011 (UTC)
- Wickey-nl should take a break. Note that the article Valence (chemistry) is also compromised. Resonance (chemistry) has been rescued (for now) V8rik (talk) 23:25, 11 March 2011 (UTC)
"Protons" and "hydrogen ions" in biochemical parlance
Hello!
It is known that words "protons" and "hydrogen ions" are preferred by biochemists rather than terms used for H+ in chemistry. This parlance appears in such terms as "proton pump". Because they study nothing but aqueous solutions, this certainly means an aqueous form of H+. Of course, it is their established terminology and it is correct to put such titles as "proton pump" to Wikipedia, as there are no protons pumps in chemistry nor in physics. But some of them are convinced that articles "proton" and "hydrogen ion" in Wikipedia are valid targets for what their papers refers as such. An advice appreciated, how to deal with edits[2] by biochemists. There are hundreds of such links, and these must be fixed in the future. Incnis Mrsi (talk) 22:16, 13 March 2011 (UTC)
- You make excellent points that the term hydron is technically preferable to proton. The usage of proton is, however, extremely common in chemistry. For example, hydron is not used in textbooks that I consult. In Wikipedia, our first mission is to be readable and understandable, which sometimes means that we use imperfect nomenclature that is better understood than what IUPAC recommends. I agree with your dislike of hydrogen ion and would support merger of hydrogen ion and hydron. These are just one editor's views, of course. Thanks for asking the community. --Smokefoot (talk) 00:10, 14 March 2011 (UTC)
- There may be various nomenclatures: IUPAC (oxonium/hydron), traditional (hydronium/proton/hydrogen ion), layman's (simply "acid"). If numerous scientists like the word "proton", then it may be an acceptable wording in articles about aqueous chemistry, but not in the "proton" wiki form, because its primary meaning is a sub-atomic particle which cannot behave as a component of an aqueous solution. It is not important which title will have an article about "proton" as biochemists understand it. It is important that there should be linkable articles (may be even more than existing two: hydron (chemistry) and hydronium) which consider the topic, expected by a context, not such things as QCD properties of nucleons or hydride anions. Incnis Mrsi (talk) 10:37, 14 March 2011 (UTC)
- As a possible solution I can propose to create a new article hydrogen cation in aqueous solution with complete coverage of this topic (including biochemistry), make several handy redirects to it like H+(aq), make "hydrogen ion" an explicit dab page with three choices: •hydron, •H+(aq), •hydrogen anion, and divert all biochemical "protons" and "hydrogen ions" to "H+(aq)". Thank you for attention. Incnis Mrsi (talk) 10:55, 14 March 2011 (UTC)
This isn't a big deal. Why are you so concerned? Most hydrons are protons. Just slap a section in proton, describing the fact that it's used to mean H+(aq) as well as 1H+(g) and as a component in atomic nuclei (several protons and neutrons).
Ben (talk) 12:18, 14 March 2011 (UTC)
- I've added a hatnote to Proton pointing to Hydronium as the aqueous biochemistry version of the term, and a sentence to the lead of Hydrogen ion to similar effect. Hopefully these will help allay any confusion. Antony–22 (talk⁄contribs) 19:00, 14 March 2011 (UTC)
Some feedback would be appreciated here. 08:07, 15 March 2011 (UTC)
Chemistry for nuclear reactors
It would be timely to collect basic material relevant to the problems with the nuclear reactors in Japan. For the formation of hydrogen - is this from H2O + Zr to give ZrO2 + H2? The hydrides of zirconium may be relevant to embrittlement of fuel rod cladding. Zirconium(II) hydride has no references and is thin. Hydrogen embrittlement has almost no useful overview/textbook references. Fukushima I Nuclear Power Plant has little chemistry explained either. There are probably other topics that would be sought by readers as they try to understand this unfolding disaster.--Smokefoot (talk) 14:05, 15 March 2011 (UTC)
- I've started scrambling something at Zirconium hydride (maybe not exactly what you outlined - little time to research the subject). Materialscientist (talk) 08:16, 16 March 2011 (UTC)
We should improve the zircaloy article, with all that is being mentioned about the failure modes of the nuclear uranium fuel rod cladding. 184.144.160.156 (talk) 09:38, 16 March 2011 (UTC)
- Improved a bit. Materialscientist (talk) 07:59, 18 March 2011 (UTC)
- Good points to start an expansions would be:
- Neeb, Karl-Heinz (1997). The radiochemistry of nuclear power plants with light water reactors. p. 487. ISBN 9783110132427.
- Parry, G. W; Lowe, A. L (1984). Zirconium in the Nuclear Industry.
- Hofmann, P (1999). "Current knowledge on core degradation phenomena, a review". Journal of Nuclear Materials. 270 (1–2): 194–211. doi:10.1016/S0022-3115(98)00899-X.
- Sherman, M (1984). "Hydrogen combustion in nuclear plant accidents and associated containment loads☆". Nuclear Engineering and Design. 82 (1): 13–24. doi:10.1016/0029-5493(84)90263-2.
- Lewis, B; Dickson, R; Iglesias, F; Ducros, G; Kudo, T (2008). "Overview of experimental programs on core melt progression and fission product release behaviour". Journal of Nuclear Materials. 380 (1–3): 126–143. doi:10.1016/j.jnucmat.2008.07.005.
- "Chemical-physical behavior of light water reactor core components tested under severe reactor accident conditions in the CORA facility".
--Stone (talk) 10:11, 16 March 2011 (UTC)
Residence time
Can someone please explain the quantitative relationship, in chemical kinetics, between "residence time" and rate constant. Maybe create a stub? I'm preparing an article on Metal ions in aqueous solution and my sources use the term in relation to rate of exchange beween coordinated water molecules and bulk solvent. What is the relationship between residence time and half-life? Petergans (talk) 14:47, 16 March 2011 (UTC)
- The residence time is the inverse of the rate constant or the half-life divided by ln(2). --Leyo 15:33, 16 March 2011 (UTC)
- Thanks. Incredible as it might seem I could not find this anywhere. Atkins, Phys. Chem., uses the term "time constant" with the same meaning. Petergans (talk) 15:54, 17 March 2011 (UTC)
Anyone seen this recently? References seem excessive. --Rifleman 82 (talk) 09:41, 22 March 2011 (UTC)
Crisscross method (of analytical chemistry?)
Hi Chemists!
The stub "crisscross method" is an orphan (lacking links from other articles) without any references. The stub claims to describes a method for naming chemicals.
If the topic is trivial, then it shouldn't be on WP. If it is nontrivial, then please consider adding at least one reference.
Thanks! Regards, Kiefer.Wolfowitz (Discussion) 17:30, 25 March 2011 (UTC)
- I've added a source and external link...it's an introductory method of naming certain chemicals. Smallman12q (talk) 17:51, 25 March 2011 (UTC)
- Thanks! I placed "about" templates on both pages: crisscross method and criss-cross algorithm. I also left the baby article on your doorstop, rating it low importance and stub class. Thanks again, Kiefer.Wolfowitz (Discussion) 18:05, 25 March 2011 (UTC)
NMR
Is there a place in {{chembox}} to add NMR images? A number of them are being uploaded but not used anywhere.Smallman12q (talk) 17:51, 25 March 2011 (UTC)
- These should be shown on the data page, as we do with things like IR data - see Acetic_acid_(data_page) for an example. Normally we have only given numerical data rather than full spectra, though I personally wouldn't object to full spectra. Walkerma (talk) 04:18, 26 March 2011 (UTC)
Hyphens in science article names
We need to have a certain position on this general issue (better say, I would like to know an answer :). Please comment here. Materialscientist (talk) 00:25, 26 March 2011 (UTC)
Reactionbox image-size
I was just trying to add a {{reactionbox}} to vinylcyclopropane rearrangement, but the File:Vinylcyclopropane rearrangement general.png image is too large to fit. I don't see a ImageSize= parameter for that template to specify how large to render the Image= filename, and my one feeble attempt broke many things. Could someone more familiar with this template take a peek? DMacks (talk) 19:06, 22 March 2011 (UTC)
- Gave up! I did get this edit to work {{#if:{{{Image|}}}|[[Image:{{{Image}}}|250px]]<br />, OK if you don't mind a fixed width. Ronhjones (Talk) 22:52, 30 March 2011 (UTC)
Self-Assembled Monolayers
I am new to editing wikipedia, so I'm not clear on how to go suggesting/making changes, but I am an assistant professor with appointments in chemistry and physics and I would like to help out with what limited time I have. How should I proceed?
More to the point: the page on Self-Assembled Monolayers is factually incorrect. Silanes are listed as forming SAMs, but they form self-organized monolayers, not self-assembled monolayers. As is correctly stated in Wikipedia's own page on self-assembly, the constituent processes of self-assembly must be reversible. Silanes (and siloxanes) form covalent bonds that can not be considered reversible under the conditions used to form monolayers. The same applies to most forms of electro and electroless deposition.
In general, the page on self-assembled monolayers conflates monolayers and self-assembled monolayers; the difference is non-trivial and the two should not be presented as if they were identical.
I made some small edits to the SAM page. I also added much-needed references to the early work on SAMs of alkane thiolates--the current article relies far too much on Love et al. wich is a review of SAMs as a form of nanotechnology.
ChezChemistry (talk) 05:23, 31 March 2011 (UTC)
π (pi)
The usage of Π is under discussion, see Talk:Pi. Since π is also pi bond, I thought I'd let you know. 65.93.12.101 (talk) 03:11, 4 April 2011 (UTC)
Can some chemists take a look at this new article please? I can't figure out if it's promotional material, or actually useful and encyclopedic but just in need of clean-up. Thanks, --Physics is all gnomes (talk) 16:29, 9 April 2011 (UTC)
Category for disputed chemical structures
Whereas Template:Low quality chem categorizes into Category:Chemical structure quality issues, images tagged with Template:Disputed chem are currently found in subcategories of Category:Accuracy disputes. I think it should have its own category. What about Category:Disputed chemical diagrams (similar to category name on Commons)? --Leyo 09:55, 9 March 2011 (UTC)
- Sounds OK. But I would also suggest we should be looking to move all diagrams to commons anyway - is there ever going to be a need to have a structure drawing on en-wiki? Ronhjones (Talk) 20:43, 9 March 2011 (UTC)
- We have a legacy of chemical structures on en-wiki (a small fraction). Only good ones should be moved to Commons. In the case of a disputed file (accuracy or quality) a replacement should be uploaded to Commons. --Leyo 21:43, 9 March 2011 (UTC)
- It's a bigger fraction than you thought. There are a lot of uncategorised structures on en-wiki, that have been hiding away. I'm trying to add categories to them so they can be listed and checked. In 2 evenings I've done all the numbers, and half way through the "A"s in the hidden category - Category:All free media. I've put the PD ones into Category:PD chem (277 currently), and any that were CC-BY-SA into Category:Chemical structures (88 currently), I suspect there are a lot more... Ronhjones (Talk) 01:16, 2 April 2011 (UTC)
- You got me wrong: I meant that the files in that category represent a small fraction of all chemical structures on en-wiki.
- There is quite some work to make the triage (moving to Commons, re-drawing or deleting). I started nominating a bunch of low quality and orphaned files for deletion (Wikipedia:Files for deletion/2011 April 2). --Leyo 01:27, 2 April 2011 (UTC)
- Ah, OK. Anyway, I'll keep adding cats when I have some spare time - there's plenty of poor unused structures out there. Ronhjones (Talk) 21:28, 2 April 2011 (UTC)
- It's a bigger fraction than you thought. There are a lot of uncategorised structures on en-wiki, that have been hiding away. I'm trying to add categories to them so they can be listed and checked. In 2 evenings I've done all the numbers, and half way through the "A"s in the hidden category - Category:All free media. I've put the PD ones into Category:PD chem (277 currently), and any that were CC-BY-SA into Category:Chemical structures (88 currently), I suspect there are a lot more... Ronhjones (Talk) 01:16, 2 April 2011 (UTC)
- We have a legacy of chemical structures on en-wiki (a small fraction). Only good ones should be moved to Commons. In the case of a disputed file (accuracy or quality) a replacement should be uploaded to Commons. --Leyo 21:43, 9 March 2011 (UTC)
I adapted the template and created Category:Disputed chemical diagrams. --Leyo 19:36, 23 March 2011 (UTC)
- I adjusted {{Disputed diagram}} populate that same cat. (it's specifically for chem diagrams, despite its generic-sounding name) DMacks (talk) 20:41, 23 March 2011 (UTC)
- There are only 4 in the category at present, 3 have WP:FfD in progress, so will probably go, as for the forth one (File:Oxygen evolving complex.png) - it's not my field. Ronhjones (Talk) 20:35, 25 March 2011 (UTC)
- And we even have 2 templates categorizing in this category: Template:Disputed chem and Template:Disputed diagram. I would suggest to merge the latter into the former, because one is enough and its name is very unspecific (not mentioning chemistry). --Leyo 20:51, 25 March 2011 (UTC)
- Support - Template:Disputed diagram often gets non chemical drawings put into it. Ronhjones (Talk) 22:00, 30 March 2011 (UTC)
- Support. DMacks (talk) 06:29, 3 April 2011 (UTC)
- OK, merged. Can Template:Disputed diagram get deleted now? --Leyo 18:59, 3 April 2011 (UTC)
- → Wikipedia:Templates for discussion/Log/2011 April 9 --Leyo 15:08, 9 April 2011 (UTC)
- And we even have 2 templates categorizing in this category: Template:Disputed chem and Template:Disputed diagram. I would suggest to merge the latter into the former, because one is enough and its name is very unspecific (not mentioning chemistry). --Leyo 20:51, 25 March 2011 (UTC)
- There are only 4 in the category at present, 3 have WP:FfD in progress, so will probably go, as for the forth one (File:Oxygen evolving complex.png) - it's not my field. Ronhjones (Talk) 20:35, 25 March 2011 (UTC)
Leyo, the community gave you the mop for a reason. Instead of clogging up FFD, why don't you just prod them and delete them if unopposed for the requisite period of time? --Rifleman 82 (talk) 00:38, 3 April 2011 (UTC)
- I am sorry, but I do not understand the term “prod”. The bunch of files in nominated for deleted were not tagged as disputed. --Leyo 18:59, 3 April 2011 (UTC)
- Wikipedia:Criteria_for_speedy_deletion. Files - F1 - redundant. You could hang a Wikipedia:Proposed deletion tag on it for a week and if nobody objects, delete it. --Rifleman 82 (talk) 19:04, 3 April 2011 (UTC)
- WP:PROD is not for images. We could certainly make a case for that being a sub-par policy decision (this is a great example) and there are ways around it, but it's what we currently have here on .en. I'm sure someone must have written an interface gadget that simplifies the FFD nomination process (like the "Nominate for deletion" toolbox item on commons). DMacks (talk) 19:45, 3 April 2011 (UTC)
- Twinkle does. --Leyo 21:36, 3 April 2011 (UTC)
- All the en-structures - I am managing to look at 1 letter a day in hidden Category:All free media - so I've done the numbers, A, B, C, and D (I guess I've added about 1200 structures so far now in Category:Chemical structures or Category:PD chem!), I'll do E tonight. Then a 2-3 day break :-) - It's easier for me to add just the categories quickly with HotCat than anything else - rather than spending time looking at each one - and there are some duplicates that don't always come one after the other.. My idea to move forward is
- Once the entire list oif images has been scanned...
- I was then going to create a new category (temporarily) and add all the files to it with AWB
- I (or maybe some help from others?) can screen this list, and remove all the good ones - easy to to with HotCat, just click the (-) sign.
- Then we'll have a category list of structures for deletion - How we delete them - I think we can decide when the list is finalised and we have agreement (one can always use transverse in TW to quickly go though a category)
- Does all that sound reasonable? There's no great rush as it will take me at least to May to run through all 450,422 images in All free media! Ronhjones (Talk) 19:54, 8 April 2011 (UTC)
- Yes, sounds reasonable. One question: According to which criteria do you decide whether you put an image to Category:Chemical structures or Category:PD chem? IMHO the latter category should better be reserved for {{PD chem}}. Hence, I would suggest to use the former category.
- There are four possibilities (without accuracy disputes):
- In use, good quality → move to Commons (Category:Chemical structures to be moved to Commons?)
- Orphaned, good quality and not redundant to another file → move to Commons (Category:Chemical structures to be moved to Commons?)
- In use, low/poor quality → to be re-drawn (tag with {{Low quality chem}})
- Orphaned, low/poor quality → to be re-drawn (tag with {{Low quality chem}} or directly nominated for deletion)
- --Leyo 15:08, 9 April 2011 (UTC)
- If they are already some sort of PD, then I use "PD chem", if they are CC-BY-SA then "chemical structures", although as we know you cannot really copyright a simple structure. Anyway, I have to find them first... When I have found them all, I'll come back here. Ronhjones (Talk) 22:52, 10 April 2011 (UTC)
- All the en-structures - I am managing to look at 1 letter a day in hidden Category:All free media - so I've done the numbers, A, B, C, and D (I guess I've added about 1200 structures so far now in Category:Chemical structures or Category:PD chem!), I'll do E tonight. Then a 2-3 day break :-) - It's easier for me to add just the categories quickly with HotCat than anything else - rather than spending time looking at each one - and there are some duplicates that don't always come one after the other.. My idea to move forward is
- Twinkle does. --Leyo 21:36, 3 April 2011 (UTC)
- WP:PROD is not for images. We could certainly make a case for that being a sub-par policy decision (this is a great example) and there are ways around it, but it's what we currently have here on .en. I'm sure someone must have written an interface gadget that simplifies the FFD nomination process (like the "Nominate for deletion" toolbox item on commons). DMacks (talk) 19:45, 3 April 2011 (UTC)
- Wikipedia:Criteria_for_speedy_deletion. Files - F1 - redundant. You could hang a Wikipedia:Proposed deletion tag on it for a week and if nobody objects, delete it. --Rifleman 82 (talk) 19:04, 3 April 2011 (UTC)
Adopt an orphan
Monopolin has been an orphan protein since the winter of 2009. "Mono" means "lonely" in Greek... Will spring 2011 bring hope? You can make a difference. walk victor falk talk 11:15, 10 April 2011 (UTC)
Coming articles
As in previous years, I advise some young, mainly one-time editors in writing articles for Wikipedia. These short articles will be appearing over the coming few weeks. Most articles will be new, but a some will be expansions of sections of existing reports. So far as I can tell, all compounds are noteworthy, to some extent. Here's the list of what is supposed to be coming: NbOCl3, CoCp*2, triisobutylaluminium, lithium 12-hydroxystearate, tellurol (expand), EuS, hemithioacetal, dithiopyridinedicarboxylic acid, PhSO2H, t-butylthiol, FeCp*2, C6Me6, Yersiniabactin, 1,4-bis(hydroxymethyl)cyclohexane, Dimethylol ethylene urea (spacing in name?), di(2-ethylhexyl)phosphoric acid, K2TaF7, t-BuCP, ferroverdin, Al2Et3Cl3, SF5Cl, Ph2Ppy, selenourea, Ni(dmgH)2, Ph2C=S, CrO2F2, 2-ethylanthraquinone (expand), hair dye (expand chem including diaminotoluene), S2, Cp2V, cycloheptanone (expand), bis(trimethylsilyl)acetylene, azidocoumarin, hexamethylbenzene. I will link these as they appear. --Smokefoot (talk) 17:07, 29 March 2011 (UTC)
- File:TBuCP-from-xtal-3D-balls.png and Commons:Category:Thiobenzophenone exist - might be of use. Good luck, some of these will be interesting articles.
- I hereby agree to do janitorial work to make these articles presentable. I need a few weeks though.--Smokefoot (talk) 02:52, 31 March 2011 (UTC)
- If only the Michigan professors were not so lazy! --Rifleman 82 (talk) 03:36, 31 March 2011 (UTC)
- I hereby agree to do janitorial work to make these articles presentable. I need a few weeks though.--Smokefoot (talk) 02:52, 31 March 2011 (UTC)
- Rather than hurl insults...perhaps you could engage in productive dialogue with those who have the same goals as you: Improving the science content on Wikipedia. UMChemProfessor (talk) 12:56, 13 April 2011 (UTC)
There are no insults. Post a message on this talk and we shall help where we can. Materialscientist (talk) 13:03, 13 April 2011 (UTC)
- I disagree - there is a small problem that the Michigan teachers dont do much followup work. Look at the edits by the the instructor - UMChemProfessor. What, no editing of content? Look at the history of Insertion reaction, which was inflicted on Wikipedia by this group - very mediocre content, some of which was just wrong. If teachers force their students to contribute content, the teacher also must edit, usually after the student have left the course. But we dont see that kind of participation do we? Hence the complaints.--Smokefoot (talk) 13:22, 13 April 2011 (UTC)
Request Help with Merge
When we tried to properly move the sandbox version to the live site, my students spelled Plasma Polymerization instead of Plasma polymerization (lower case 'p'), which resulted in a new site (duplicate) being created. We requested the merger of the two sites last Friday and have seen no action. Can someone help us speed up this request? Or give us some advice on how to best handle the two sites. Thanks! UMChemProfessor (talk) 13:43, 12 April 2011 (UTC)
- Moved, merged history. Materialscientist (talk) 13:57, 12 April 2011 (UTC)
Isotopes_and_half-life.svg – colors
I just noticed that User:CharlesHBennett added a note to File:Isotopes_and_half-life.svg saying that 40Ca should be colored stable, and User:Linefeed subsequently made the requested change, both of them without notifying me.
I think this is a bad idea. The colors in the image were generated algorithmically from the Nuclear Wallet Cards database, and if there is a problem with the algorithm, it should be fixed and the whole image regenerated. There shouldn't be a special rule for 40Ca.
If I remember correctly, I only used the color labeled "stable" for isotopes that are listed as "STABLE" in the database. The listed half life of 40Ca is "3.0E+21 Y GT", so it got the darkest red. I thought at the time that it was worth making a distinction between theoretically stable and theoretically unstable (but very long lived) elements. I may be wrong, though. Does anybody have a suggestion or a preference? I could, for example, replace "stable" with "> 1015 yr" and blacken things accordingly, continuing to ignore "GT" and "LT" and error bars. Some isotopes have a listed half life of "60 NS GT", so I think that it's not a good idea to treat everything with "GT" in the lifetime as stable.
I suppose this matters very little, but the current patch-up job bugs me. -- BenRG (talk) 21:17, 14 April 2011 (UTC)
- Very basic question, can you just confirm that this is correct: "We are 100% sure that a 40Ca atom will eventually decay (given infinite time), based on E=mc^2 analysis. But we have never seen one decay because its lifetime is so long." Is that right? --Steve (talk) 03:29, 15 April 2011 (UTC)
- Maybe you can ask at WP:PHYSICS? Don't think we have any nuclear chemists here... --Rifleman 82 (talk) 03:32, 15 April 2011 (UTC)
- The border between stable and unstable isotope is vague and depends on the current ability to measure long half-lives. The best example is perhaps bismuth-209 which is considered unstable with a half-life of 2e19 yr, but this doesn't mean 2e19 yr is this border. Yes, we don't decide it and take "stable" from sources, but. "3.0E+21 Y GT" is way beyond measurable, and thus it is a problem of that database if they consider calcium-40 unstable. Materialscientist (talk) 04:05, 15 April 2011 (UTC)
- Maybe you can ask at WP:PHYSICS? Don't think we have any nuclear chemists here... --Rifleman 82 (talk) 03:32, 15 April 2011 (UTC)
In answer to Steve, the excellent Wikipedia articles stable isotope and list of nuclides list and give the modes of decay of the many nuclides that are theoretically unstable by mc^2 analysis, but practically stable because they have such long half-life that they have never been observed to decay. Calcium 40 is one of these, and should theoretically decay given infinite time. When I wrote my comment, I had not realized that Calcium 40 was theoretically unstable---I had wrongly supposed it was absolutely stable. I think this is an important distinction to preserve, so I would recommend returning to the original color scheme favored by BenRG, where nuclides such as calcium 40 are colored differently from absolutely stable ones like oxygen 16. Sorry for having created this confusion in the first place.
In answer to Steve, the excellent Wikipedia articles stable isotope and list of nuclides list and give the modes of decay of the many nuclides that are theoretically unstable by mc^2 analysis, but practically stable because they have such long half-life that they have never been observed to decay. Calcium 40 is one of these, and should theoretically decay given infinite time. When I wrote my comment, I had not realized that Calcium 40 was theoretically unstable---I had wrongly supposed it was absolutely stable. I think this is an important distinction to preserve, so I would recommend returning to the original color scheme favored by BenRG, where nuclides such as calcium 40 are colored differently from absolutely stable ones like oxygen 16. Sorry for having created this confusion in the first place. I still have a question possibly needing advice from a nuclear expert. Is Calcium 40 theoretically capable of decay by double electron capture (as the stable isotope article says, or by double positron emission as the list of nuclides says, or both? I strongly suspect both, based on the following reasoning. Double positron emission is listed as having a decay energy of 0.194 MeV, whereas if the two positrons then annihilated two electrons, an additional 2.044 MeV of annihilation gamma rays would be produced, resulting in a total energy release of around 2.24 MeV for decay by double electron capture, so energetically both decay modes should be possible.CharlesHBennett (talk) 05:12, 15 April 2011 (UTC)CharlesHBennett (talk) 05:21, 15 April 2011 (UTC)
Doubly-labelled water
Doubly-labeled water is up for renaming. At issue is whether there should be a hyphen or not. 65.94.45.160 (talk) 05:20, 17 April 2011 (UTC)
Student project that could really use some help
Hello, I think the article Nitrogen Flow through Metabolism is a student project, but it seems like the article isn't a very well defined topic and could really use some additional input on how it should be named/advice on if it should be moved somewhere. If anyone could provide help that would be great! I am not sufficiently steeped in this kind of biology to make a good call, Sadads (talk) 13:03, 17 April 2011 (UTC)
Peer review on DNA nanotechnology articles
Hi all! I currently have peer reviews running for two articles I have worked on, DNA nanotechnology (review page) and Nucleic acid design (review page). I'd appreciate it if you could take a look and leave any feedback you could give. Thanks! Antony–22 (talk⁄contribs) 01:41, 19 April 2011 (UTC)
Two University Chemistry Project
We have two University Chemistry Project editing chemistry articles.
- The first one (538) with 11 students working on Cationic_polymerization, Polyfluorene, Polymer_brush, High Refractive Index Polymers, Plasma_polymerization.
- The other (215) with 72 students working on Appel reaction, Ritter reaction, Jones oxidation
Most of the editing will be done in sandboxes and the end copied to the article space from what I see. The some work can be viewed by this two links:
I tried to get in contact with some of the participants, but up to now there was no reaction. A hit and run editing of the page looks likely. From the what I can extract from the history of those projects the possibility that anybody will become an regular wikipedia editor is 0%. What should we do?
- Do nothing: Than we have 8 long and most likely good article with little to some work to clean them up and make them consistent with the others we already have.
- Try to help as friend: Try hard to get into contact and positively influence the creation of the article.
- Try to help like a wikilawyer: We have enough guidelines we can pour over them to show that working with the community helps.
- Strafing the infantry: Work on the 17 articles ourself and see what happens when they try to replace them.
I would like to go with 2. suggestion, but for this I would need some help from the rest of the Chemistry Project.
--Stone (talk) 15:49, 16 February 2011 (UTC)
- (2) informally is definitely the best way to go - it may even improve the odds from 0% :) I'll try to drop by now and then to the sandboxes (though ATM the organization is abit chaotic for me to understand).
- I am a little cautious about too much help though, since it is supposed to be their work (while it sits in the sandbox). We should, however, enlist substantial help from the organizers (UMChemProf/GS) to help in the clean-up effort. Jon C (talk) 19:57, 16 February 2011 (UTC)
- Jon C and Stone, thank you very much for your comments, as ChemProf has said below we are very much trying to give the community a chance to comment and would very much like you to help us out with this as there really is not a common place for the "rules" other than the Manual of Style which the students do use. (if that is different and has changed, it might need some updating). As Jon C mentioned, we do need to evaluate the students so if you could leave a majority of the work to the students that would be much appreciated. However, if you wanted to edit one thing as an example so that the students better understand what you are requesting of them, that might be fine. They now each have their individual usernames and therefore we can differentiate their edits from yours. However, i do want to stress that we would like the students to do a majority of the work. Also please give them a heads up that you have made a change so that they can do a comparison. They all should sign up with their email addresses so you can contact them this way. MichChemGSI (talk) 01:55, 14 April 2011 (UTC)
- I would like to help them in a way that the clean up is not substantial. Like al the boring MOS things and that they do not step on one of the hidden tripwires in wikipedia like 3RR, NPOV, COI, .....--21:11, 16 February 2011 (UTC)
- I recommend a blend of options 2 and 3. We will be dealing with this problem forever until we can point to guidelines. Overall, we offer to help and guide but we indicate (insist) that the standard mechanism of editing pages is incremental and consensus driven, which facilitate debate and revisions. We are here not to serve courses but the readership, not a class at a school.
- Further thoughts:
- Of specific and urgent need is a response to these editors intentions of adding a lot of spectroscopy into articles on reactions. I am sure that learning about how spectroscopy can be used to follow the Appel reaction, I am do not think that this information is appropriate for Wikipedia. If others feel similarly, we should communicate our thoughts before they spend too much time writing up an essay on the theme.
- I for one like the crisp format and content of many of the articles on named reactions. Easy to read and get the main message. I anticipate that when these school projects are complete, the "improved" articles will be more thorough but less approachable. Perhaps an inevitable consequence of "progress." Somehow the idea is current that long article are intrinsically superior than short ones.--Smokefoot (talk) 00:47, 17 February 2011 (UTC)
- Responding to the comments above, I edited our Manual of Style with respect to how we edit, which I indicate is done incrementally and is guided by consensus, and the inclusion of analytical data, which are often not sought. I figured that I would be bold and make these recommendations, but as usual, these recommendations are subject to everyone's comments.--Smokefoot (talk) 00:41, 18 February 2011 (UTC)
I am the chem professor who initiated and is overseeing the graduate class project, centered on the editing of polymer-related sites. I would like to remind you all that I brought this project to your attention here earlier this year and asked for feedback throughout the term. This term I put all the sandboxes in a central location so that the community can find them easily and comment on the development of each site. We received several comments and have addressed them all. The students are responding to the feedback.
I would like to remind the new community members that I have been revising the project each year in response to comments from the community. You can find previous discussions here 1, and 2, and 3. For example, all users now have their own username. This term we are "moving" the sites to the real page instead of copy/paste as in past years, to keep an accurate record of the history.
In response to the other criticism, many of my students continue to contribute to Wikipedia after the term is over. In fact, the first student club for Wikipedia was founded at Michigan by one of my graduate students. Michigan Student Club Many undergrads are now involved as well. My graduate student is a Wikipedia Student Ambassador continues to be actively involved with Wikipedia and will be presenting at the next Wikimania.
If you have further comments/suggestions, please leave me a message at my talk page. UMChemProfessor (talk) 17:58, 8 April 2011 (UTC)
Getting the most out of student projects
Hey folks. It seems like everyone shares the same goal of improving Wikipedia's chemistry articles, but the there's a bit of tension around what constitutes improvement and what improved articles about chemical reactions ought to look like. Maybe it would help if we tried to come to a common agreement about what great articles of the type these students are working on should look like. If there's disagreement, for example, on how much spectroscopy should figure into coverage, then we should try to build a consensus about the appropriate type of spectroscopy content. Thoughts? Have any WP:CHEM regulars looked into how the students' work has shaped up so far this term?--Sage Ross - Online Facilitator, Wikimedia Foundation (talk) 14:31, 13 April 2011 (UTC)
- Thanks for helping out. To my mind the tension is between what is good for Wikipedia - excellent content - and what is good for the teachers - a convenient medium for writing essays. In particular, no matter how good the students are, their articles require some "janitorial work": getting better references, redrawing, reformatting. We never see this effort from Michigan, despite their posturing and requests for advice.
- Referencing. No one can fault these young editors for not coughing up enough references. There is excessive reliance on primary (US-oriented) literature vs a balance according to WP:SECONDARY - see brush polymers.
- SpectroscopyI think that the consensus here with this project is that spectra and spectral data are usually inappropriate for this encyclopedia.
- Topic selection. Often colleges slice and dice areas into very narrow themes to suit their need to write essays. Example Oxo wall. These topics are contrived to meet the needs of the class vs the goals of Wikipedia.
I revised our MOS in response to the Michigan work, but this advice is not followed (or debated). See Wikipedia:Manual of Style (chemistry)#Scope In any case, editors here would welcome a discussion of standards and expectations. --Smokefoot (talk) 17:55, 13 April 2011 (UTC)
- Role of the instructor
If the instructor wishes to use Wikipedia as a platform for class work, then he should take greater ownership of teaching the students how to edit. And that begins by being an editor first. He would thus be familiar with community standards and customs, such as by participating in community discussions about how articles should look like. After some time here, he would be able to guide the students better.
Ink has been spilled on Wikipedia users and their reputations (e.g. how this affects their interactions with others). In this case, an instructor who is an active editor here of good standing will avoid many pitfalls, but more than that, get more positive attitudes and support from the community than one who has no reputation (= no contributions).
- Editing style
It appears that many school groups prefer to work on articles in sandboxes. The existing editors here simply don't know about it - they only find out about it one day when the article goes "live". While this might be convenient in terms of grading or other trivial details, it deprives these editors the opportunity to be acclimatized to the prevailing standards, either through editors copyediting their work, or by editors leaving comments, suggestions, and pointers to the relevant MOS subpages on article talk pages. All this happens in one go shortly after the article goes live, and the students might feel that their work is mangled beyond recognition, and that leads to the revert wars mentioned previously.
On occasion, these student sandbox-editors decide to overwrite existing work wholesale. This upsets the present editors, because it is disrespectful to the original contributors, it is rude and contrary to Wikipedia's collaborative style, and it usually requires a great deal of cleanup. The important things at the bottom of the page (interwiki, categories, etc.) are often lost as well.
Now if the students were to edit existing articles outside of sandboxes, it might be difficult to tell the students' work from the other editors'. What's good for the instructor may not be good for Wikipedia.
- Communication
The students are usually non-communicative. For example, above (#University of Michigan Honors Organic Chemistry Project, they asked for comments, but after editors here spent time and effort giving comments... there were no replies. In other examples, when these usually non-communicative students do communicate, they are combative and refuse criticism. It's probably not surprising that you got comments like Strafing the infantry: Work on the 17 articles ourself and see what happens when they try to replace them. (Stone). The editors here are somewhat frustrated with student groups in general, maybe a little more so for those from Michigan (user reputation = social capital). --Rifleman 82 (talk) 23:26, 13 April 2011 (UTC)
- these students in the Honors Organic Chemistry Project have been notified of the comments. however they have not exactly been given in a manner that the students can tease out what the consensus is. They do read the comments and are trying to address what they can. You need to give them a bit of time as they are working on finals at the moment and cannot get to them right away. But if your comments could be more concise and specific and if another community member disagrees then, then it is difficult to take action on the comment. We do very much respect your comments and feedback, but again when comments are in disagreement, we cannot make both parties happy. Thank you. MichChemGSI (talk) 01:42, 14 April 2011 (UTC)
- It's not exactly reviewer comments which need to be answered in one fell swoop, all or nothing. Think of it as ping pong. Student works on it a little, editor here comments a little, rinse lather repeat. Either way, an "okay, thanks" would be acknowledgment rather than giving editors the feeling that they are asking for comments for comments' sake. That was three weeks ago, by the way. --Rifleman 82 (talk) 02:02, 14 April 2011 (UTC)
- these students in the Honors Organic Chemistry Project have been notified of the comments. however they have not exactly been given in a manner that the students can tease out what the consensus is. They do read the comments and are trying to address what they can. You need to give them a bit of time as they are working on finals at the moment and cannot get to them right away. But if your comments could be more concise and specific and if another community member disagrees then, then it is difficult to take action on the comment. We do very much respect your comments and feedback, but again when comments are in disagreement, we cannot make both parties happy. Thank you. MichChemGSI (talk) 01:42, 14 April 2011 (UTC)
Hi, I would like to add that perhaps the Chem editing community should revise the A rating or perhaps even a FA rating for a Chemistry article. This semester we had our students look at the A rated articles to get an idea for a goal in mind. In reference to your viewpoint of long articles vs short articles, do short articles get an A rating? If we can work together towards creating guidelines to the A rating for chemistry articles then the students and all of us would know what constitutes and excellent article. Currently the chemistry rating page is way below par it does not give any indication for a good article. We have one for chemicals http://en.wikipedia.org/wiki/Wikipedia:WikiProject_Chemicals/Assessment. but i feel that this is out of date and not very specific http://en.wikipedia.org/wiki/Wikipedia:WikiProject_Chemistry/Assessment/A-class_review. Basically i feel that we as a community need to revise our Assessment system and update it so that we all can help to improve the Chemistry content here on Wikipedia. We can work on the assessment this summer before the courses start so that we (Michigan and other university communities) know what the Wikipedia community wants to see in a Chemistry FA. Thoughts? MichChemGSI (talk) 02:44, 14 April 2011 (UTC)
- Response
I would like to address some of the comments above: First, this project (at Michigan) was never intended to be collections of essays. From day 1, our goal was to make quality Wikipedia articles on Chemistry topics. I admit that it has taken us awhile to learn the "rules" -- written and otherwise -- about how to construct a quality article. And it seems from these discussions, there is still some disagreement on what a quality entry looks like. I welcome further discussion on this topic, and will revise the project as needed to meet these goals.
Regarding the "cleaning" up of articles after my students post them, if you would let us know what the "problems" are...I can address them next year. I was not aware of major re-editing of the student content once they go live. Yes, if I edited pages myself, then maybe I would learn all those tricks. However, I simply don't have the time to devote to substantial editing at this point in my career. Whenever we learn of requirements, like adding DOI's, we incorporate that into the next round of the project.
Regarding topic selection, I have a few comments. First, many of my student select topics off of the WP:CHEM page which identifies articles that could use help. Second, after the first term (when I first learned of WP:CHEM) I have now always posted the topics selected at the beginning of the term and solicited feedback. Never has anyone commented on our topic selection. Third, in only a handful of instances, have new sites been created. Most of the time they are revising current sites.
Regarding references, my students are now being graded on finding balanced (and secondary) sources. Again, this is something I've learned is important through WP:CHEM and am now incorporating into the project.
Regarding editing in sandboxes, this is still most convenient for this project, because the students work in teams and throughout the term. However, this year I have created a central location for all the sandboxes (my user page) and have alerted you folks to this at the beginning of the term. In addition, the students post on the current site that they are working on it during the term and the give the location of the sandbox. We got feedback on several of those sites and have responded to all of it. So you do have a voice "during" the editing process. Although we used to copy/paste the sandboxes, I was made aware of the "move" feature this year, and we now use that. This way the editing history and discussion are also moved.
If you look back at all the discussions about this project, you will see that it has evolved quite substantially, and I am excited to continue evolving it. However, I must admit, the hostility that we have been receiving of late, makes me question whether I want to further pursue this endeavor. The students love the project, I love that the science content is expanding/improving, and I know many users are appreciative of our work. My colleagues have also been excited by this project, which is why it has expanded to include other courses here at Michigan (Inorganic Chemistry (507), Materials Chemistry (511) and now the undergraduate honors organic chemistry (215HH)). Many of these professors do not even have a Wikipedia account, but would like to use it in the classroom. Thus, MichChemGSI and myself try to assist these courses, but it is not the same as having direct oversight and contact with the students. So, though you may "blame" us for being lazy or posturing, we really are trying our best. UMChemProfessor (talk) 21:36, 14 April 2011 (UTC)
- Don't mind the naysayers, that's just part of the territory around here! From my experience, WP:CHEM is a bit segregated from the larger Wikipedia community. The Manual of Style (chemistry) is just an underdeveloped guideline and consensus can always change. My recommendation is to focus your energy in sticking to the key policies, which are much more hashed out. Consider that there are larger goals here than what WP:CHEM imagines. Many times, Wikipedia is in need of someone being bold. If you want to know what quality articles look like, take a look at some featured ones in the sciences to get some inspiration. I had a frustrating time getting started here at perfluorooctanoic acid (mostly from just one editor in this project). Many others, however, are very helpful. I am glad to see you here. From my perspective, we need and want you here. =) As the Guardian headline reads, Wikipedia wants more contributions from academics. Shootbamboo (talk) 22:52, 14 April 2011 (UTC)
- @UMChemProfessor: your work is appreciated, please continue. Don't hesitate (ask your students) to post question - as Shootbamboo mentioned, many WP:Chem rules are unspoken, simply because they are undeveloped or nobody wrote them down yet. One way to learn could be go through the article history, look for "cleanup" edits (by WP:Chem people) and ask why this and that correction was made. Materialscientist (talk) 23:28, 14 April 2011 (UTC)
- Some of the tidying which needs to be done includes fixing the case of the section headings (which are not title case, unlike many other places, and which try to avoid repeating the article title MOS:HEAD), wikilinking to and from other relevant articles (although wikilinks should not lead to the userspace), and having low-quality images which have poorly chosen filenames (figure 1.png is not meaningful, and does not help its re-use), have English language text (use the captions for that) which prevent re-use in other language Wikipedias. It's not difficult work but it's tedious. I'm sure you're busy, but I would still encourage you to edit Wikipedia when time permits, because you will understand how it works more than by talking about it. --Rifleman 82 (talk) 23:11, 14 April 2011 (UTC)
- I guess that I disagree with the soothing words to Michigan instructors. Students forced to write articles are not academics - they are students forced to write essays. Second problem, last time I checked, we dont serve the Guardian. The problem with the Michigan group is lack of faculty involvement. To be sweet to these forced editors and lauding to their teachers - such flattery is easy and feels good but the rhetoric is hollow. But our mission is to an encyclopedia, not making people feel good. Some pointed guidance could advance the encyclopedia. Feel-good talk does that less well. Oh well.--Smokefoot (talk) 13:01, 15 April 2011 (UTC)
General comment, not directed at Michigan - to all WP chemists: I might quit WP:CHEM if we don't start getting strict. Our laissez-faire attitude annoys me no end. We should block more editors who waste our time (categorisation, nomenclature) and we shouldn't be so tolerant and inclusive. We should focus on quality, not letting everyone help. I wouldn't mind if I were hassled by WP:CHEM editors for making mistakes or acting too unilaterally or foolishly. We do need more editors, but only ones who write exceptionally well. I guess I disagree with Wikipedia's inclusiveness. Maybe it shouldn't be the encyclopedia that anyone can edit. It should be restricted (not necessarily to "experts" but) to those that can toe the line and make high quality stuff. I know this is an unpopular view, but maybe that's because everyone else who shares it avoids Wikipedia. Man up, Wikichemists.
Ben (talk) 16:49, 15 April 2011 (UTC)
- UMChemProfessor said the students are now being graded on finding secondary sources. So I think it's disparaging to characterize them as students being forced to write essays, and not articles. Our mission includes not biting the newcomers. We do serve Wikipedia and the Guardian just highlighted a mission of ours. But pointed advice always has its place. Of course the professors should make sure the students are writing well. It's a college assignment after all. Shootbamboo (talk) 21:54, 15 April 2011 (UTC)
- Not you too Ben! =) If people are disruptive, they should get a block, as they do here, consider WP:ANI for disruptive users. Topic bans can also be applied. I think, over time, people that don't cut it get weeded out. But yes, our model makes incorporating newcomers a perennial to-do here. This is what, the 8th most popular website in the world? Not too shabby. But yes, I see quality issues as paramount. Shootbamboo (talk) 22:28, 15 April 2011 (UTC)
Yeah, I disagree with not biting the newcomers. We should eat them whole unless they've got potential and are receptive to instructions.
In my experience, editors who are not vandals but who pursue their own agenda in defiance of the will of WP:CHEM are not sanctioned soon enough or hard enough (in fact, we rarely do more than complaining on talk pages). The problem with Wikipedia's chemisty articles is inconsistency. How well a topic is covered seems to have very little to do with its importance.
Rhodocene, lovely article that it is, just isn't an important chemical in the way that glucose or carbon dioxide are. Maybe we pay more attention to systematic analysis, draw some pie charts, and get a better feel of the relative importance of the topics within our project's scope. Martin Walker gave me some very useful links to article stats:
I myself am guilty of putting too much effort into unimportant articles, because they're easier to make good and you're less likely to be overwritten. But we've got to improve, and university projects don't seem to be helping much.
Ben (talk) 10:54, 18 April 2011 (UTC)
- First, Ben - as noted above, the "will" of WP:CHEM is not clear in this case. Some contributors are very supportive of our efforts, and some are strongly against it. I don't view us as "pursuing our own agenda in defiance of the will of WP:CHEM" because we are constantly asking for feedback on the project and each term, I revise the project in accordance to the "majority" opinion on each matter. Second, I think your point about focusing on "important" articles is a good one. The question I want to put out there for discussion is who do we consider as the audience when deeming an article "important"? I would argue there are two users: (1) The obvious one is the general public (who are accessing the sites in the numbers you indicate above). (2) But what about the chemistry community (undergrads, grads, faculty, industry)? Aren't they also important (though outnumbered) users of WP:CHEM content? Shouldn't we also strive to have high-quality and useful content available for them? UMChemProfessor (talk) 00:36, 19 April 2011 (UTC)
Hi UMChemProfessor,
Firstly, as I wrote in bold at the beginning of my first comment, these comments are not directed at Michigan. I was actually thinking of certain editors who have been obsessed with categorisation. And I agree that the will of WP:CHEM is not always clear, but I would like it to be clear. We need more backbone, and less tolerance of bad editing. I do not wish us to simply 'close the door' to any new contributors. On the contrary, I'd like us to train wannabe Wikichemists to our own high standards. But I want us make sure new editors edit to our satisfaction before we let them loose on our articles.
Secondly, I agree that it's not simply a numbers game. We can have general and technical content side-by-side, and treat both as equally important. I made my plea because many of us (including me) have focussed too much on the latter. The significant difference between general readers and the chemistry community is the latter (particularly beyond undergraduates!) should have easy access to better information than Wikipedia, and are thus less dependent on us. It's all very well having ideals, but we must be pragmatic in allocating our resources. We have limited time and effort and should work to make Wikipedia of maximum overall benefit to society, within its remit.
One thing Michigan could do to make collaboration with WP:CHEM easier is to make small changes to articles at a time. For example, a student could focus on adding a few extra key details and properly formatted references to a synthesis section of an article about a compound. They could add their content to that section, and we'd all have a few days or weeks to see if it's OK or needs modification. Lots of little edits like this are the best way to work together. Your previous approach of saying things along the lines of "we've got a new draft for such and such an article, please comment on it otherwise we'll replace the current article with our draft in two days' time" is difficult for us, because it demands we read a large amount of work and make detailed comments in a short time frame. Make it easier for us, and that'll make it easier for you and your students.
Ben (talk) 09:30, 19 April 2011 (UTC)
- Ben, Thanks for helping move the discussion forward in a productive manner! I am interested in continuing this project, as are other professors here at Michigan. It would be great if we could come to a consensus on many of the topics discussed above. I like the idea of having the students "practice edit" some pages (maybe even the page they will work on in a substantial way) in order to get them "versed" in the best practices early in the semester. Though I will not be teaching a graduate course this Fall, I will likely be involved (from a distance) with at least one graduate course which incorporates this project. If you (broader you) have any other suggestions, I am open to them. With regards to topics, I think it will be difficult for us to ask the students to edit chemical pages in a graduate course (though maybe they could practice on one of these). But I can encourage them to select topics off of the WP:CHEM page listing. So managing that list can help focus efforts on those topics deemed more "important" than others. UMChemProfessor (talk) 17:11, 23 April 2011 (UTC)
In a way, all edits are practice edits. I recommend your students edit the same way that all of us regulars edit - bit-by-bit. Just do things a bit at a time. What's difficult about graduates editing chemical pages? You're welcome to propose new topics that are not on any current lists, but just get agreement here that such topics are important enough. It's all so much easier when done in an integrated, communicative way. --Ben (talk) 18:10, 23 April 2011 (UTC)
Anyone knows the difference? Some sources hyphenate them. Conditions appear similar if not identical. --Rifleman 82 (talk) 03:32, 22 March 2011 (UTC)
My boss, Bob Moriarty worked for Sarett, and if you look at the seminal work in JACS- 1953, you find that Sarett actually dropped CrO3 into pyridine. That's it. Isolation of the products by extraction usually. Whereas, the seminal work of Collins- TL 1968 reads that they found dichloromethane. Dissolve alcohol into DCM then add the pyridine-CrO3 complex. MKMkinch2 (talk) 23:23, 23 April 2011 (UTC)
Thank you! Tojo's book [3] seems to indicate gradual development of CrO3-pyridine reagents. Perhaps they should all be combined into a single article since they are quite similar. I don't expect much more development in this direction in view of the unpopularity of Cr reagents.
That aside, welcome to WP Chemistry. If you have questions or need any help, feel free to drop a note here. Happy editing! --Rifleman 82 (talk) 23:50, 23 April 2011 (UTC)
University of Michigan Honors Organic Chemistry Project
Over the course of this winter semester (Jan-April) students enrolled in the University of Michigan Honors section of Organic Chemistry II will be editing three current named reactions sites, the Ritter reaction, the Appel reaction and Jones oxidation. For each page, the students will be adding four sections, history, animation, spectroscopy and applications. The history section will give a brief background about the reaction. The animation section will provide a detailed animation of the mechanism for this reaction and we hope to add a sound track to accompany each step. The spectroscopy section will highlight the major changes that will occur in the NMR spectra during the course of the reaction. Finally, the applications section will describe how this reaction is used today in various chemical settings. The SSG leaders listed at the bottom of our userpage will be facilitating this project and the students will be creating their rough drafts in our sandboxes. No edits will be posted until the final page is completed at the end of the semester. — Preceding unsigned comment added by UmichSSG215 (talk • contribs) 18:34, 2 February 2011 (UTC) — Preceding unsigned comment added by UmichSSGleader (talk • contribs)
- History, mechanism, and modern applications sound like great topics...easy to focus on the specific reaction (i.e., the topic of the article) but also to cross-link it to preceding methodologies (we probably have articles on some of them) and associated reactions--build the web rather than just a self-contained lit-review. I don't think extensive commentary on the NMR changes in the article about a reaction is especially worthwhile content for wikipedia though. It's a great demonstration that a student understands the structural changes occurring, but it doesn't seem to help anyone else understand anything about the reaction beyond the fact of those structural changes. Seems like this would be more attuned to a self-contained paper on the reaction rather than an integrated encyclopedia, where spectral features of a functional group are universal for the functional group (for which we likely have a specific page) rather than being (in the logical conclusion) cut'n'pasted into every reaction that has that functional group as a reactant or product. DMacks (talk) 04:51, 9 February 2011 (UTC)
- I agree with DMacks here. Spectroscopic changes are here generally out of place (or they must be really universal for all reactions - like the disappearance of a C=O vibration in the IR after reduction to an alcohol, note that the NMR signals of neighbouring C-H groups are so dependent on other substituents as well that they are indicative, but hard to generalise, and note that some of these are so obvious that the description does not fit in an encyclopedia - for the IR C=O vibration one could maybe mention that the reaction can easily be followed by IR, but that is about as much detail as necessary here).
- I also want to make a point regarding detailed animations of mechanisms. I am against that, it is difficult to stop them at an appropriate point to be able to follow them, a normal written out mechanism is generally more illustrative as key-steps in a mechanism can then be more easily studied or shown. For chemists an animation may be easy to follow (but even, some rearrangements are pretty difficult, so would need a slow speed), but for non-chemists they are completely useless, and that should be the aim of writing here, so that the article and all the information is also suitable for the general public. Also, Wikipedia is not an entertainment site, I don't think that sounds are a good plan either. --Dirk Beetstra T C 07:52, 9 February 2011 (UTC)
- I also ask that you and your students listen to editors here. In the past, established editors here at Wikipedia have often experienced difficulty dealing with the Michigan's student-editors, whom I have found to be both arrogant and misguidedly determined to push their perspective, perhaps because these students seek article ownership as some sort of grading scheme. As you can see above, some of us are wary of animations and certain kinds of data. We work by consensus here. So please listen.--Smokefoot (talk) 13:45, 9 February 2011 (UTC)
- Great idea , I am looking forward to the results. I have some pointers as well. Try not to replace an article but expand an article. For example the Ritter reaction (mostly created by the outstanding User:~K) is short but good quality and its content should not be replaced. I think the animations (see the discussion @Talk:Electrophilic_aromatic_substitution#addition_of_animation) are a great initiative. Animations can be much more informative than cartoons. However it may very well be that a certain animation does not stay published for long when a majority of editors feel it lacks quality or relevance. Same goes for the spectroscopy ( by the way: Wikipedia barely has any spectroscopic data at all!). I have collaborated on Holton Taxol total synthesis with people from St. Olaf College Minnesota and one of the things they did was adding a section on protective groups. Not exactly encyclopedia style but the result is very enlightening and should stay. Also try to give each user its own user account (no group accounts!) and more free advise: consider grading students based on edits and not based on a single article. If a students spots a big error and fixes it, that should be rewarded. V8rik (talk) 18:40, 9 February 2011 (UTC)
Hi Everyone, our additions to the Ritter reaction page are starting to take shape over at User:UmichSSGleader/Ritter Reaction, where we have been working on them. We would welcome any suggestions you might have about our work if anyone is interested in checking it out. Bear in mind that this is not complete yet (obviously, since an entire section is still blank), but please feel free to comment on what is done. —Preceding undated comment added 01:37, 23 March 2011 (UTC).
- Here are some thoughts I had about your draft
- Thanks for asking our opinions – this is a welcome approach
- Well done on retaining the existing article's content, which is good
- You could improve the current article by replacing primary literature citations with review article citations. For example, for the range of alcohols that can be used as alkylating agents, surely you can cite one or two reviews for the whole range rather than a different primary paper for each alcohol. As an encyclopaedia like Wikipedia is a tertiary source, we should mainly cite secondary sources (textbooks and reviews) rather than primary sources (original articles).
- "In May of 1948, P. Paul Minieri, Ritter's student, submitted a thesis to the Graduate School of New York University in order to fulfill the requirements for the degree of Doctor of Philosophy" would be better if shortened to "Minieri submitted work on the reaction as his PhD thesis".
- Do readers really want or need to know the old-fashioned method Ritter & Minieri used to prove their reaction worked? I'm not against it in principle, I just wonder if it's too much (unimportant) detail for a short article.
- You say "The Ritter reaction is most useful in the formation of new carbon-nitrogen bonds" - what else is it useful for, apart from making C-N bonds? Or by "most useful" do you mean 'really useful"? If so, find a reference for that value judgement.
- "Real world applications" is a colloquial phrase that might best be replaced with "industrial applications" or "large-scale applications"
- "A problem with the Ritter reaction is the necessity of an extremely strong acid catalyst in order to produce the carbocation. This poses an environmental risk, as the acids are extremely corrosive and also cannot be reused" - again, need a reference for this seemingly alarmist view of H2SO4, arguably the most important chemical in industry (made on the largest scale worldwide). It's only an environmental risk if it escapes. From the previous paragraph, it seems the Ritter reaction's main industrial application is in pharmaceutical synthesis, so we're talking much smaller scale than commodity chemicals and thus much less of an environmental concern purely from a scale POV. Isn't a bigger issue the fact that strong acid makes the reaction unsuitable for delicate substrates, limiting scope?
- Your animation of the mechanism is OK, but a bit jerky. Curly arrows in a different colour, maybe red, would be good. The imine intermediate is drawn in an unlikely high-energy linear conformation, should be bent (sp2 nitrogen). The final product amide is not drawn in the preferred conformation - should have tBu and carbonyl oxygen syn: Acta Cryst. (2004). E60, o448-o449, Bull. Chem. Soc. Jpn. (1994) 67, 1226–1231.
- Ben (talk) 20:23, 23 March 2011 (UTC)
- Correction: there does in fact not exist a ban on using peer-reviewed articles. Primary literature is really someone's lab notebook. Secondary literature is peer-reviewed articles and tertiary literature are the handbooks and reviews. Love the animation, would prefer a slight pause when the arrows are in view V8rik (talk) 20:31, 23 March 2011 (UTC)
- It is rubbish to say that "Primary literature is really someone's lab notebook" Jeesh. Inexperienced editors tend to rely too heavily on primary journals, in part because such citations are easier to find and, such citations somehow make some folks feel that that they are more in touch (such that they have the insights to choose from the many thousand of research papers appearing each year in the chemical literature). Knowledge of the secondary sources is difficult because locating them requires more breadth and more skill. Specialization is easier than general knowledge. And Wikipedia should aim for general knowledge. We should always urge editors to lean toward general sources of information, although doing so exclusively is impractical.--Smokefoot (talk) 00:27, 24 March 2011 (UTC)
- V8rik: I didn't say there's a ban, I said we prefer reviews and books. Primary/secondary/tertiary have different meanings to different people, that's why I stated my definition. You often hear talk of "primary literature" meaning peer-reviewed articles. Ben (talk) 20:55, 23 March 2011 (UTC)
- understood! V8rik (talk) 21:21, 23 March 2011 (UTC)
- My view is that research articles (whether you count them as primary or secondary) and reviews are both permissible, but serve different purposes. Research articles establish verifiability but not things like neutral point of view, proper balance, or notability. Review articles are much better at satisfying the latter three, but since they tend be less in-depth than the original literature, they are somewhat less useful for verifying details. It is important to cite both types of literature in order to satisfy both verifiability and NPOV/balance/notability. Antony–22 (talk⁄contribs) 03:30, 24 March 2011 (UTC)
- understood! V8rik (talk) 21:21, 23 March 2011 (UTC)
- Regarding the animation, is it based on actual (calculated or measured) positions and timing, or just an application of general mechanistic ("first-principles") ideas? Either way, the description-page needs to clarify the origin (either citing the factual basis for the specifics or confirming that it's just a hypothetical/conceptual illustration). If the latter, would be better if each step were more discrete (avoiding motion for one step beginning until the previous step has ended). For examples, the nitrile is already coming in (and attracting the eye) while the carbocation is still forming, and then the water is approaching even before the N is fully +1 charge. DMacks (talk) 20:35, 23 March 2011 (UTC)
- It's generally fine. I wonder what you can say in "Spectroscopy" without being overly specific, and without veering into original research. Why the inconsistent fonts in the animation? Would be nice if you can discuss the industrial examples mentioned in some detail. Perhaps something from OPRD? {{cite journal}} and Doi Bot are helpful. --Rifleman 82 (talk) 20:36, 23 March 2011 (UTC)
Jones oxidation
Hey everyone, my page on Jones oxidation is also coming together. Take a look at it here. It's definitely still a work in progress and we're still working on the editing process, but please leave comments if you have any. UMich215SSG (talk) 01:22, 28 March 2011 (UTC)
An extended discussion of Jones is not warranted. Such details should go into his article (which exists in this case). I would only keep "The reaction was first discovered by ERH Jones in 1946 when he was working with preparing acetylenic ketones. [1]Oxidation of secondary alcohols via Jones' oxidation was discovered in 1949 in continuation of the experiments with acetylenic ketones. [2]"
Sections should not start with "the". IR, 1H NMR, and 13C NMR spectra are of low quality. The presence of this section does not seem to fit the article, but it seems it was a required section by your advisor, whatever that means. If you must, you should speak in generalities and not spend too much time on it as it is really quite minor. The changes are centered on the presence/absence of spectroscopic signals attributed to C=O - 1H NMR ~ 2.5 ppm or so, 13C NMR ~ 200 ppm, and IR ~ 1700-1800 cm^-1. Examples are fine but should not take up so much space - stack the spectra, with structures. Colors are helpful in this case. Either way, you should generate them as PNGs, not scan them.
Comments with regard to the animation are the same as that mentioned earlier, though it is less jerky which is good.
Remember not to have a space between the element name and the oxidation state. Scribd is not a reliable source. The Jones oxidation works most of the time, so do choose your examples with care. One illustrative bench-scale reaction would be nice, but WP is not a manual, so describe the synthesis but don't write it like an experimental section. The aspirin example is interesting and can be expanded. There is a move away from chromium reagents so I won't expect modern industrial use otherwise.
Once again, do read WP:CHEMMOS. That's the style guide we work by. Have fun. --Rifleman 82 (talk) 03:29, 28 March 2011 (UTC)
- Thanks for the feedback! We made some changes per your advice. Most of the history was moved to ERH Jones' page. We ended up not using real spectra due to issues getting permissions and making them easy to read, though I do think some IR examples might be useful if they were touched up in such a way to be helpful. We changed the ChemDraw formatting of the mechanism as specified in the manual of style. I plan on moving the page to the real site very soon since we are at the end of our project. However, I do plan to stick around if more editing is necessary (and will encourage my students to do the same).UMich215SSG (talk) 01:33, 15 April 2011 (UTC)
- I see your article has gone live, congratulations. Some things remain to be fixed, appreciate it if you can look at it.
- History should be de-peacocked. If someone can find a WP:RS which says he's the best chemist of his generation, then cite that. If not, I think we should only keep the last two sentences. If there are only two sentences kept, it should be merged into the lead section.
- Think about removing English language text from the image so that it can be re-used in other language wikis. That's the primary motivation for uploading to commons. Is one of the curly arrows straight? Is this a proposed or accepted mechanism, or are the intermediates actually observed? I don't expect anyone to go out and prove it but just stating it is helpful.
- It's nice to have the mech. section but I think you should rename it "reaction" or something, and state explicitly early on that primary alcohols give carboxylic acids, and secondary alcohols give ketones. And now this is how it happens: etc. I think the spectroscopy section should be folded in there too because that section doesn't say much either. "The synthesis can be monitored by ν(CO) in IR spectroscopy, and the ... in 1H NMR spectroscopy. 13C NMR spectroscopy is probably too slow to be useful to "follow" reactions routinely.
- "Chromium" is not a proper noun, so it is not capitalized when it is not the first word of a sentence
- Section headers are in sentence case, not title case
--Rifleman 82 (talk) 14:39, 19 April 2011 (UTC)
- Note that almost all of these concerns are standard Manual of Style guidelines, universal for all of wikipedia. DMacks (talk) 16:12, 19 April 2011 (UTC)
- Regarding the spectroscopy section, I agree that it's overdone. It's also got numerous mistakes (at least incorrect suggestions and incorrect terminology, not outright falsehood). For example, do chemists actually usually monitor the progress of the reaction, or just analyze the product after-the-fact? IR 3600 cm-1 is the O-H bond, whereas an "alcohol functional group" is defined to also include the C-O bond. That other bond has a different frequency, and is also an important change to observe in the IR--doesn't a carboxylic acid also have an O-H bond? Isn't that a significant confirmation that the oxidation has gone both steps rather than stopping at the aldehyde stage? According to the 1H section, this spectroscopy is only useful for comparing alcohol to acid...not for secondary alcohols? Alcohol hydrogens are extremely variable and sometimes hard to find, not a reliable signal at all--seems like this whole section was written as just generic extraction of data from standard correlation tables (which again is why many of us think it's got too much generic content). Seems like a critical (and less variable) change is the disappearance of the carbinol hydrogen (that does work also for oxidation of secondaries). Most of my students usually forget that this H is actually one of the most important parts of the entire reaction. "The hydrogen in the alcohol functional group of a carboxylic acid functional group gives a peak between 10.5 - 12 ppm. Due to electron withdrawing effects of the carbonyl, the hydrogen is not observed on the H-NMR spectra." That's both self-contradictory--how can it give a peak if it's not observed?--and uses incorrect technial wording--a carboxylic acid is definitely not an alcohol. DMacks (talk) 16:27, 19 April 2011 (UTC)
- Rifleman and DMacks, thanks you for your feedback and sorry that I was unable to respond to it sooner. It appears that Smokefoot has helpfully come in and made various changes. I agree that a spectroscopy section was inherently challenging due to its specific nature, but the other people in our project wanted to give it a shot at least. The only thing I disagree with is the extreme truncating of the Mechanism Section. The arrow pushing mechanism was removed (I believe because Smokefoot thought it looked too messy/cumbersome?) and also the animation was removed. I can see the argument that the Chemdraw image of the mechanism is cumbersome, and could perhaps be abbreviated, but I still think it is useful enough to be included. And I also think the animation should be added back (I can remove the English from the animation as Rifleman suggested). What does everyone else think? UMich215SSG (talk) 23:42, 24 April 2011 (UTC)
CrO3 under normal conditions is not monomeric like depicted. I can't speak for Smokefoot but many inorganic chemists are very uncomfortable with drawing curly arrows because the range of probable mechanisms are quite different for inorganics, and it is actually quite difficult to distinguish them. I personally did not appreciate the video, and I think many others above were not quite enthusiastic. I'll leave Smokefoot to discuss his specific comments about the mechanism. --Rifleman 82 (talk) 00:16, 25 April 2011 (UTC)
- Yes I reworked Jones oxidation and Appel reaction today. The big arrow-pushing schemes in Jones oxidation seemed like a homework assignment (I used to do that kind of stuff for homework), but you'd never see that kind of contortions in an encyclopedia/monograph/textbooks. And it had the problems that students often do - inorganic species are depicted in silly ways (monomeric three-coordinat Cr(IV)). I just re-read the section in March, previously uncited, which is excellent (after 6 editions you'd think so). The Mechanism section did not discuss mechanism (at all!), but March emphasized the giant isotope effect (6?) for the alpha hydride shift and the role for Cr(VI), Cr(V), and Cr(IV). There was no stoichiometry shown, which seems like a serious oversight for a reagent article. The many references to old Jones and Djerassi papers are almost irrelevant to a reader seeking info, so I move them to a sep section. No mention was made of other Cr(VI) reagents, still a gap. For the Appel reaction, the main thing was to move the biography of Rolf Appel. It would be nice to bolster his bio, because he did other nifty things with P=C bonds. The Michigan work did helped organize my thoughts and readings. They are welcome to edit my work of course! For example, my equations are still not balanced! --Smokefoot (talk) 03:43, 25 April 2011 (UTC)
Why does the Jones oxidation proceed via negatively charged intermediates in a strongly acidic environment? I'd expect it to go via positively charged and neutral intermediates, such as those shown in this book, this other book, this website and this other website.
Ben (talk) 12:17, 25 April 2011 (UTC)
- I dont know why the ester is often drawn as an anion, but I dont know the pKa's of CrO2(OH)2, it might be lower than sulfuric. The anionic half ester is like MeOSO3-, I guess. I was unable to balance the equations. The equations in potassium permanganate are even more messed up. And no attempt to show stoichiometry is given in the cumbersomely named Oxidation of primary alcohols to carboxylic acids or Oxidation of secondary alcohols to ketones. --Smokefoot (talk) 13:03, 25 April 2011 (UTC)
I agree with Ben, the anionic mechanism is unfamiliar to me, I have only seen positively or neutrally charged intermediates. I also think mechanisms typed in a word processor are harder for the eye to follow and that a more simplified structural mechanisms might be more clear. For example, something like this:
This mechanism is also more in line with mechanisms I've seen in the literature. Is there some kind of consensus established for preference of typed mechanisms over structure images? UMich215SSG (talk) 13:18, 25 April 2011 (UTC)
- Well let's try find some sources for the issue at hand:
- what is the Cr(VI) species that forms the initial "chromate ester" intermediates. As a general source, March's "Advanced Organic Chemistry.." cites Augustyn (sp?)'s book on organic oxidations.
- Jan Rocek was a prominent contributor who isolated the first Cr(V) ester ("Synthesis of stable chromium(V) complexes of tertiary hydroxy acids"JACS 1979, 101 , pp 3206. doi:10.1021/ja00506a013, cited 111 times, which is a good sign).
- I am not a big fan of arrow pushing, especially with metals. Concise ChemDraw is often better than line equations. But there is no ambiguity with the structure for ROCrO3-, so why draw it out? I dont think that it helps to show details of a potential acid-catalyzed route to this mixed ester. The key to chromate oxidations is the scission of the C-H bond, not the ester formation.
- Organic chemists need to as careful with the Cr part at they insist inorganickers be with the C part. There is probably no CrO3H-.
- BTW, Petergans wrote a nice article on chromic acid with estimates of the first pKa.--Smokefoot (talk) 13:47, 25 April 2011 (UTC)
- Well let's try find some sources for the issue at hand:
There are many sources that show and legitimize the acid catalyzed mechanism that I showed above. The sources Ben listed: here, here, here and and here. As well as Bruckner's Organic Mechanisms: Reactions, Stereochemistry, and Synthesis pp. 750-752 and Ege's textbook Structure and Reactivity (I don't have the page numbers off hand). I suppose it doesn't matter which one is described on the page, but I think it warrants at least mentioning this mechanism.
Also thanks for taking the time to add to the mechanism section, largely it's content has been improved substantially. However, I still think line equations and written descriptions are harder to follow than structural diagrams. You can argue that it seems redundant to you, but not everyone who views this page necessarily has or is pursuing a Chem PhD. Wikipedia should still be informative to non-experts and students. I am in no way suggesting that we "dumb down" anything, but ChemDraw mechanisms would could supplement text and add clarity. Arrow pushing can be minimized to reflect accuracy and reduce clutter. I'd be happy to make these myself (might take me a few days though). UMich215SSG (talk) 15:24, 26 April 2011 (UTC)
The chromic acid mechanism in Reusch seems more reasonable since you are using water and sulfuric acid in your system than the "CrO3" which is not ordinarily monomeric. Unless these species are observed, probably best to prefix it with "a suggested mechanism". --Rifleman 82 (talk) 16:03, 26 April 2011 (UTC)