A fundamental physical constant occurring in quantum mechanics is the Planck constant h . A common abbreviation is ħ = h /2π reduced Planck constant or Dirac constant .
Quantity (Common Name/s)
(Common) Symbol/s
Defining Equation
SI Units
Dimension
Wavefunction
ψ, Ψ
To solve from the Schrödinger equation
varies with situation and number of particles
Wavefunction probability density
ρ
ρ
=
|
Ψ
|
2
=
Ψ
∗
Ψ
{\displaystyle \rho =\left|\Psi \right|^{2}=\Psi ^{*}\Psi }
m−3
[L]−3
Wavefunction probability current
j
Non-relativistic, no external field:
j
=
−
i
ℏ
2
m
(
Ψ
∗
∇
Ψ
−
Ψ
∇
Ψ
∗
)
=
ℏ
m
Im
(
Ψ
∗
∇
Ψ
)
=
Re
(
Ψ
∗
ℏ
i
m
∇
Ψ
)
{\displaystyle {\begin{aligned}\mathbf {j} &={\frac {-i\hbar }{2m}}\left(\Psi ^{*}\nabla \Psi -\Psi \nabla \Psi ^{*}\right)\\&={\frac {\hbar }{m}}\operatorname {Im} \left(\Psi ^{*}\nabla \Psi \right)=\operatorname {Re} \left(\Psi ^{*}{\frac {\hbar }{im}}\nabla \Psi \right)\end{aligned}}}
m−2 s−1
[T]−1 [L]−2
The general form of wavefunction for a system of particles, each with position r i sz i . Sums are over the discrete variable sz , integrals over continuous positions r .
For clarity and brevity, the coordinates are collected into tuples, the indices label the particles (which cannot be done physically, but is mathematically necessary). Following are general mathematical results, used in calculations.
Property or effect
Nomenclature
Equation
Wavefunction for N particles in 3d
r = (r 1 , r 2 ... r N sz = (s z 1s z 2sz N )
In function notation:
Ψ
=
Ψ
(
r
,
s
z
,
t
)
{\displaystyle \Psi =\Psi \left(\mathbf {r} ,\mathbf {s_{z}} ,t\right)}
in bra–ket notation :
|
Ψ
⟩
=
∑
s
z
1
∑
s
z
2
⋯
∑
s
z
N
∫
V
1
∫
V
2
⋯
∫
V
N
d
r
1
d
r
2
⋯
d
r
N
Ψ
|
r
,
s
z
⟩
{\displaystyle |\Psi \rangle =\sum _{s_{z1}}\sum _{s_{z2}}\cdots \sum _{s_{zN}}\int _{V_{1}}\int _{V_{2}}\cdots \int _{V_{N}}\mathrm {d} \mathbf {r} _{1}\mathrm {d} \mathbf {r} _{2}\cdots \mathrm {d} \mathbf {r} _{N}\Psi |\mathbf {r} ,\mathbf {s_{z}} \rangle }
for non-interacting particles:
Ψ
=
∏
n
=
1
N
Ψ
(
r
n
,
s
z
n
,
t
)
{\displaystyle \Psi =\prod _{n=1}^{N}\Psi \left(\mathbf {r} _{n},s_{zn},t\right)}
Position-momentum Fourier transform (1 particle in 3d)
Φ = momentum-space wavefunction
Ψ = position-space wavefunction
Φ
(
p
,
s
z
,
t
)
=
1
2
π
ℏ
3
∫
a
l
l
s
p
a
c
e
e
−
i
p
⋅
r
/
ℏ
Ψ
(
r
,
s
z
,
t
)
d
3
r
↿⇂
Ψ
(
r
,
s
z
,
t
)
=
1
2
π
ℏ
3
∫
a
l
l
s
p
a
c
e
e
+
i
p
⋅
r
/
ℏ
Φ
(
p
,
s
z
,
t
)
d
3
p
n
{\displaystyle {\begin{aligned}\Phi (\mathbf {p} ,s_{z},t)&={\frac {1}{{\sqrt {2\pi \hbar }}^{3}}}\int \limits _{\mathrm {all\,space} }e^{-i\mathbf {p} \cdot \mathbf {r} /\hbar }\Psi (\mathbf {r} ,s_{z},t)\mathrm {d} ^{3}\mathbf {r} \\&\upharpoonleft \downharpoonright \\\Psi (\mathbf {r} ,s_{z},t)&={\frac {1}{{\sqrt {2\pi \hbar }}^{3}}}\int \limits _{\mathrm {all\,space} }e^{+i\mathbf {p} \cdot \mathbf {r} /\hbar }\Phi (\mathbf {p} ,s_{z},t)\mathrm {d} ^{3}\mathbf {p} _{n}\\\end{aligned}}}
General probability distribution
Vj = volume (3d region) particle may occupy,P = Probability that particle 1 has position r 1 in volume V 1 with spin s z 1and particle 2 has position r 2 in volume V 2 with spin s z 2
P
=
∑
s
z
N
⋯
∑
s
z
2
∑
s
z
1
∫
V
N
⋯
∫
V
2
∫
V
1
|
Ψ
|
2
d
3
r
1
d
3
r
2
⋯
d
3
r
N
{\displaystyle P=\sum _{s_{zN}}\cdots \sum _{s_{z2}}\sum _{s_{z1}}\int _{V_{N}}\cdots \int _{V_{2}}\int _{V_{1}}\left|\Psi \right|^{2}\mathrm {d} ^{3}\mathbf {r} _{1}\mathrm {d} ^{3}\mathbf {r} _{2}\cdots \mathrm {d} ^{3}\mathbf {r} _{N}\,\!}
General normalization condition
P
=
∑
s
z
N
⋯
∑
s
z
2
∑
s
z
1
∫
a
l
l
s
p
a
c
e
⋯
∫
a
l
l
s
p
a
c
e
∫
a
l
l
s
p
a
c
e
|
Ψ
|
2
d
3
r
1
d
3
r
2
⋯
d
3
r
N
=
1
{\displaystyle P=\sum _{s_{zN}}\cdots \sum _{s_{z2}}\sum _{s_{z1}}\int \limits _{\mathrm {all\,space} }\cdots \int \limits _{\mathrm {all\,space} }\;\int \limits _{\mathrm {all\,space} }\left|\Psi \right|^{2}\mathrm {d} ^{3}\mathbf {r} _{1}\mathrm {d} ^{3}\mathbf {r} _{2}\cdots \mathrm {d} ^{3}\mathbf {r} _{N}=1\,\!}
Wave-particle duality and time evolution [ edit ] [ edit ] Summarized below are the various forms the Hamiltonian takes, with the corresponding Schrödinger equations and forms of wavefunction solutions. Notice in the case of one spatial dimension, for one particle, the partial derivative reduces to an ordinary derivative .
One particle
N particles
One dimension
H
^
=
p
^
2
2
m
+
V
(
x
)
=
−
ℏ
2
2
m
d
2
d
x
2
+
V
(
x
)
{\displaystyle {\hat {H}}={\frac {{\hat {p}}^{2}}{2m}}+V(x)=-{\frac {\hbar ^{2}}{2m}}{\frac {d^{2}}{dx^{2}}}+V(x)}
H
^
=
∑
n
=
1
N
p
^
n
2
2
m
n
+
V
(
x
1
,
x
2
,
⋯
x
N
)
=
−
ℏ
2
2
∑
n
=
1
N
1
m
n
∂
2
∂
x
n
2
+
V
(
x
1
,
x
2
,
⋯
x
N
)
{\displaystyle {\begin{aligned}{\hat {H}}&=\sum _{n=1}^{N}{\frac {{\hat {p}}_{n}^{2}}{2m_{n}}}+V(x_{1},x_{2},\cdots x_{N})\\&=-{\frac {\hbar ^{2}}{2}}\sum _{n=1}^{N}{\frac {1}{m_{n}}}{\frac {\partial ^{2}}{\partial x_{n}^{2}}}+V(x_{1},x_{2},\cdots x_{N})\end{aligned}}}
n is xn .
E
Ψ
=
−
ℏ
2
2
m
d
2
d
x
2
Ψ
+
V
Ψ
{\displaystyle E\Psi =-{\frac {\hbar ^{2}}{2m}}{\frac {d^{2}}{dx^{2}}}\Psi +V\Psi }
E
Ψ
=
−
ℏ
2
2
∑
n
=
1
N
1
m
n
∂
2
∂
x
n
2
Ψ
+
V
Ψ
.
{\displaystyle E\Psi =-{\frac {\hbar ^{2}}{2}}\sum _{n=1}^{N}{\frac {1}{m_{n}}}{\frac {\partial ^{2}}{\partial x_{n}^{2}}}\Psi +V\Psi \,.}
Ψ
(
x
,
t
)
=
ψ
(
x
)
e
−
i
E
t
/
ℏ
.
{\displaystyle \Psi (x,t)=\psi (x)e^{-iEt/\hbar }\,.}
L 2 -normbound state ) or a slowly diverging norm (if it is part of a continuum ):[ 1]
‖
ψ
‖
2
=
∫
|
ψ
(
x
)
|
2
d
x
.
{\displaystyle \|\psi \|^{2}=\int |\psi (x)|^{2}\,dx.\,}
Ψ
=
e
−
i
E
t
/
ℏ
ψ
(
x
1
,
x
2
⋯
x
N
)
{\displaystyle \Psi =e^{-iEt/\hbar }\psi (x_{1},x_{2}\cdots x_{N})}
Ψ
=
e
−
i
E
t
/
ℏ
∏
n
=
1
N
ψ
(
x
n
)
,
V
(
x
1
,
x
2
,
⋯
x
N
)
=
∑
n
=
1
N
V
(
x
n
)
.
{\displaystyle \Psi =e^{-i{Et/\hbar }}\prod _{n=1}^{N}\psi (x_{n})\,,\quad V(x_{1},x_{2},\cdots x_{N})=\sum _{n=1}^{N}V(x_{n})\,.}
Three dimensions
H
^
=
p
^
⋅
p
^
2
m
+
V
(
r
)
=
−
ℏ
2
2
m
∇
2
+
V
(
r
)
{\displaystyle {\begin{aligned}{\hat {H}}&={\frac {{\hat {\mathbf {p} }}\cdot {\hat {\mathbf {p} }}}{2m}}+V(\mathbf {r} )\\&=-{\frac {\hbar ^{2}}{2m}}\nabla ^{2}+V(\mathbf {r} )\end{aligned}}}
r = (x, y, z ).
H
^
=
∑
n
=
1
N
p
^
n
⋅
p
^
n
2
m
n
+
V
(
r
1
,
r
2
,
⋯
r
N
)
=
−
ℏ
2
2
∑
n
=
1
N
1
m
n
∇
n
2
+
V
(
r
1
,
r
2
,
⋯
r
N
)
{\displaystyle {\begin{aligned}{\hat {H}}&=\sum _{n=1}^{N}{\frac {{\hat {\mathbf {p} }}_{n}\cdot {\hat {\mathbf {p} }}_{n}}{2m_{n}}}+V(\mathbf {r} _{1},\mathbf {r} _{2},\cdots \mathbf {r} _{N})\\&=-{\frac {\hbar ^{2}}{2}}\sum _{n=1}^{N}{\frac {1}{m_{n}}}\nabla _{n}^{2}+V(\mathbf {r} _{1},\mathbf {r} _{2},\cdots \mathbf {r} _{N})\end{aligned}}}
n is r n xn , yn , zn ), and the Laplacian for particle n using the corresponding position coordinates is
∇
n
2
=
∂
2
∂
x
n
2
+
∂
2
∂
y
n
2
+
∂
2
∂
z
n
2
{\displaystyle \nabla _{n}^{2}={\frac {\partial ^{2}}{{\partial x_{n}}^{2}}}+{\frac {\partial ^{2}}{{\partial y_{n}}^{2}}}+{\frac {\partial ^{2}}{{\partial z_{n}}^{2}}}}
E
Ψ
=
−
ℏ
2
2
m
∇
2
Ψ
+
V
Ψ
{\displaystyle E\Psi =-{\frac {\hbar ^{2}}{2m}}\nabla ^{2}\Psi +V\Psi }
E
Ψ
=
−
ℏ
2
2
∑
n
=
1
N
1
m
n
∇
n
2
Ψ
+
V
Ψ
{\displaystyle E\Psi =-{\frac {\hbar ^{2}}{2}}\sum _{n=1}^{N}{\frac {1}{m_{n}}}\nabla _{n}^{2}\Psi +V\Psi }
Ψ
=
ψ
(
r
)
e
−
i
E
t
/
ℏ
{\displaystyle \Psi =\psi (\mathbf {r} )e^{-iEt/\hbar }}
Ψ
=
e
−
i
E
t
/
ℏ
ψ
(
r
1
,
r
2
⋯
r
N
)
{\displaystyle \Psi =e^{-iEt/\hbar }\psi (\mathbf {r} _{1},\mathbf {r} _{2}\cdots \mathbf {r} _{N})}
Ψ
=
e
−
i
E
t
/
ℏ
∏
n
=
1
N
ψ
(
r
n
)
,
V
(
r
1
,
r
2
,
⋯
r
N
)
=
∑
n
=
1
N
V
(
r
n
)
{\displaystyle \Psi =e^{-i{Et/\hbar }}\prod _{n=1}^{N}\psi (\mathbf {r} _{n})\,,\quad V(\mathbf {r} _{1},\mathbf {r} _{2},\cdots \mathbf {r} _{N})=\sum _{n=1}^{N}V(\mathbf {r} _{n})}
[ edit ] Again, summarized below are the various forms the Hamiltonian takes, with the corresponding Schrödinger equations and forms of solutions.
One particle
N particles
One dimension
H
^
=
p
^
2
2
m
+
V
(
x
,
t
)
=
−
ℏ
2
2
m
∂
2
∂
x
2
+
V
(
x
,
t
)
{\displaystyle {\hat {H}}={\frac {{\hat {p}}^{2}}{2m}}+V(x,t)=-{\frac {\hbar ^{2}}{2m}}{\frac {\partial ^{2}}{\partial x^{2}}}+V(x,t)}
H
^
=
∑
n
=
1
N
p
^
n
2
2
m
n
+
V
(
x
1
,
x
2
,
⋯
x
N
,
t
)
=
−
ℏ
2
2
∑
n
=
1
N
1
m
n
∂
2
∂
x
n
2
+
V
(
x
1
,
x
2
,
⋯
x
N
,
t
)
{\displaystyle {\begin{aligned}{\hat {H}}&=\sum _{n=1}^{N}{\frac {{\hat {p}}_{n}^{2}}{2m_{n}}}+V(x_{1},x_{2},\cdots x_{N},t)\\&=-{\frac {\hbar ^{2}}{2}}\sum _{n=1}^{N}{\frac {1}{m_{n}}}{\frac {\partial ^{2}}{\partial x_{n}^{2}}}+V(x_{1},x_{2},\cdots x_{N},t)\end{aligned}}}
n is xn .
i
ℏ
∂
∂
t
Ψ
=
−
ℏ
2
2
m
∂
2
∂
x
2
Ψ
+
V
Ψ
{\displaystyle i\hbar {\frac {\partial }{\partial t}}\Psi =-{\frac {\hbar ^{2}}{2m}}{\frac {\partial ^{2}}{\partial x^{2}}}\Psi +V\Psi }
i
ℏ
∂
∂
t
Ψ
=
−
ℏ
2
2
∑
n
=
1
N
1
m
n
∂
2
∂
x
n
2
Ψ
+
V
Ψ
.
{\displaystyle i\hbar {\frac {\partial }{\partial t}}\Psi =-{\frac {\hbar ^{2}}{2}}\sum _{n=1}^{N}{\frac {1}{m_{n}}}{\frac {\partial ^{2}}{\partial x_{n}^{2}}}\Psi +V\Psi \,.}
Ψ
=
Ψ
(
x
,
t
)
{\displaystyle \Psi =\Psi (x,t)}
Ψ
=
Ψ
(
x
1
,
x
2
⋯
x
N
,
t
)
{\displaystyle \Psi =\Psi (x_{1},x_{2}\cdots x_{N},t)}
Three dimensions
H
^
=
p
^
⋅
p
^
2
m
+
V
(
r
,
t
)
=
−
ℏ
2
2
m
∇
2
+
V
(
r
,
t
)
{\displaystyle {\begin{aligned}{\hat {H}}&={\frac {{\hat {\mathbf {p} }}\cdot {\hat {\mathbf {p} }}}{2m}}+V(\mathbf {r} ,t)\\&=-{\frac {\hbar ^{2}}{2m}}\nabla ^{2}+V(\mathbf {r} ,t)\\\end{aligned}}}
H
^
=
∑
n
=
1
N
p
^
n
⋅
p
^
n
2
m
n
+
V
(
r
1
,
r
2
,
⋯
r
N
,
t
)
=
−
ℏ
2
2
∑
n
=
1
N
1
m
n
∇
n
2
+
V
(
r
1
,
r
2
,
⋯
r
N
,
t
)
{\displaystyle {\begin{aligned}{\hat {H}}&=\sum _{n=1}^{N}{\frac {{\hat {\mathbf {p} }}_{n}\cdot {\hat {\mathbf {p} }}_{n}}{2m_{n}}}+V(\mathbf {r} _{1},\mathbf {r} _{2},\cdots \mathbf {r} _{N},t)\\&=-{\frac {\hbar ^{2}}{2}}\sum _{n=1}^{N}{\frac {1}{m_{n}}}\nabla _{n}^{2}+V(\mathbf {r} _{1},\mathbf {r} _{2},\cdots \mathbf {r} _{N},t)\end{aligned}}}
i
ℏ
∂
∂
t
Ψ
=
−
ℏ
2
2
m
∇
2
Ψ
+
V
Ψ
{\displaystyle i\hbar {\frac {\partial }{\partial t}}\Psi =-{\frac {\hbar ^{2}}{2m}}\nabla ^{2}\Psi +V\Psi }
i
ℏ
∂
∂
t
Ψ
=
−
ℏ
2
2
∑
n
=
1
N
1
m
n
∇
n
2
Ψ
+
V
Ψ
{\displaystyle i\hbar {\frac {\partial }{\partial t}}\Psi =-{\frac {\hbar ^{2}}{2}}\sum _{n=1}^{N}{\frac {1}{m_{n}}}\nabla _{n}^{2}\Psi +V\Psi }
[ 2]
Ψ
=
Ψ
(
r
,
t
)
{\displaystyle \Psi =\Psi (\mathbf {r} ,t)}
Ψ
=
Ψ
(
r
1
,
r
2
,
⋯
r
N
,
t
)
{\displaystyle \Psi =\Psi (\mathbf {r} _{1},\mathbf {r} _{2},\cdots \mathbf {r} _{N},t)}
Property/Effect
Nomenclature
Equation
Photoelectric equation
K max = Maximum kinetic energy of ejected electron (J)h = Planck's constant f = frequency of incident photons (Hz = s−1 )φ , Φ = Work function of the material the photons are incident on (J)
K
m
a
x
=
h
f
−
Φ
{\displaystyle K_{\mathrm {max} }=hf-\Phi \,\!}
Threshold frequency and Work function
φ , Φ = Work function of the material the photons are incident on (J)f 0 , ν 0 = Threshold frequency (Hz = s−1 )
Can only be found by experiment.
The De Broglie relations give the relation between them:
ϕ
=
h
f
0
{\displaystyle \phi =hf_{0}\,\!}
Photon momentum
p = momentum of photon (kg m s−1 )f = frequency of photon (Hz = s−1 )λ = wavelength of photon (m)
The De Broglie relations give:
p
=
h
f
/
c
=
h
/
λ
{\displaystyle p=hf/c=h/\lambda \,\!}
Quantum uncertainty [ edit ]
Probability Distributions
Property or effect
Equation
Density of states
N
(
E
)
=
8
2
π
m
3
/
2
E
1
/
2
/
h
3
{\displaystyle N(E)=8{\sqrt {2}}\pi m^{3/2}E^{1/2}/h^{3}\,\!}
Fermi–Dirac distribution (fermions)
P
(
E
i
)
=
g
(
E
i
)
e
(
E
−
μ
)
/
k
T
+
1
{\displaystyle P(E_{i})={\frac {g(E_{i})}{e^{(E-\mu )/kT}+1}}}
P (Ei ) = probability of energy Ei g (Ei ) = degeneracy of energy Ei (no of states with same energy)μ = chemical potential
Bose–Einstein distribution (bosons)
P
(
E
i
)
=
g
(
E
i
)
e
(
E
i
−
μ
)
/
k
T
−
1
{\displaystyle P(E_{i})={\frac {g(E_{i})}{e^{(E_{i}-\mu )/kT}-1}}}
Property or effect
Nomenclature
Equation
Angular momentum quantum numbers
s = spin quantum number ms = spin magnetic quantum numberℓ = Azimuthal quantum number mℓ = azimuthal magnetic quantum numberj = total angular momentum quantum numbermj = total angular momentum magnetic quantum number
Spin:
‖
s
‖
=
s
(
s
+
1
)
ℏ
m
s
∈
{
−
s
,
−
s
+
1
⋯
s
−
1
,
s
}
{\displaystyle {\begin{aligned}&\Vert \mathbf {s} \Vert ={\sqrt {s\,(s+1)}}\,\hbar \\&m_{s}\in \{-s,-s+1\cdots s-1,s\}\\\end{aligned}}\,\!}
Orbital:
ℓ
∈
{
0
⋯
n
−
1
}
m
ℓ
∈
{
−
ℓ
,
−
ℓ
+
1
⋯
ℓ
−
1
,
ℓ
}
{\displaystyle {\begin{aligned}&\ell \in \{0\cdots n-1\}\\&m_{\ell }\in \{-\ell ,-\ell +1\cdots \ell -1,\ell \}\\\end{aligned}}\,\!}
Total:
j
=
ℓ
+
s
m
j
∈
{
|
ℓ
−
s
|
,
|
ℓ
−
s
|
+
1
⋯
|
ℓ
+
s
|
−
1
,
|
ℓ
+
s
|
}
{\displaystyle {\begin{aligned}&j=\ell +s\\&m_{j}\in \{|\ell -s|,|\ell -s|+1\cdots |\ell +s|-1,|\ell +s|\}\\\end{aligned}}\,\!}
Angular momentum magnitudes
angular momementa:
S = Spin,L = orbital,J = total
Spin magnitude:
|
S
|
=
ℏ
s
(
s
+
1
)
{\displaystyle |\mathbf {S} |=\hbar {\sqrt {s(s+1)}}\,\!}
Orbital magnitude:
|
L
|
=
ℏ
ℓ
(
ℓ
+
1
)
{\displaystyle |\mathbf {L} |=\hbar {\sqrt {\ell (\ell +1)}}\,\!}
Total magnitude:
J
=
L
+
S
{\displaystyle \mathbf {J} =\mathbf {L} +\mathbf {S} \,\!}
|
J
|
=
ℏ
j
(
j
+
1
)
{\displaystyle |\mathbf {J} |=\hbar {\sqrt {j(j+1)}}\,\!}
Angular momentum components
Spin:
S
z
=
m
s
ℏ
{\displaystyle S_{z}=m_{s}\hbar \,\!}
Orbital:
L
z
=
m
ℓ
ℏ
{\displaystyle L_{z}=m_{\ell }\hbar \,\!}
Magnetic moments In what follows, B is an applied external magnetic field and the quantum numbers above are used.
Property or effect
Nomenclature
Equation
orbital magnetic dipole moment
μ
ℓ
=
−
e
L
/
2
m
e
=
g
ℓ
μ
B
ℏ
L
{\displaystyle {\boldsymbol {\mu }}_{\ell }=-e\mathbf {L} /2m_{e}=g_{\ell }{\frac {\mu _{B}}{\hbar }}\mathbf {L} \,\!}
μ
ℓ
,
z
=
−
m
ℓ
μ
B
{\displaystyle \mu _{\ell ,z}=-m_{\ell }\mu _{B}\,\!}
spin magnetic dipole moment
μ
s
=
−
e
S
/
m
e
=
g
s
μ
B
ℏ
S
{\displaystyle {\boldsymbol {\mu }}_{s}=-e\mathbf {S} /m_{e}=g_{s}{\frac {\mu _{B}}{\hbar }}\mathbf {S} \,\!}
μ
s
,
z
=
−
e
S
z
/
m
e
=
g
s
e
S
z
/
2
m
e
{\displaystyle \mu _{s,z}=-eS_{z}/m_{e}=g_{s}eS_{z}/2m_{e}\,\!}
dipole moment potential
U = potential energy of dipole in field
U
=
−
μ
⋅
B
=
−
μ
z
B
{\displaystyle U=-{\boldsymbol {\mu }}\cdot \mathbf {B} =-\mu _{z}B\,\!}
Property or effect
Nomenclature
Equation
Energy level
E
n
=
−
m
e
4
/
8
ε
0
2
h
2
n
2
=
−
13.61
e
V
/
n
2
{\displaystyle E_{n}=-me^{4}/8\varepsilon _{0}^{2}h^{2}n^{2}=-13.61\,\mathrm {eV} /n^{2}}
Spectrum
λ = wavelength of emitted photon, during electronic transition from Ei to Ej
1
λ
=
R
(
1
n
j
2
−
1
n
i
2
)
,
n
j
<
n
i
{\displaystyle {\frac {1}{\lambda }}=R\left({\frac {1}{n_{j}^{2}}}-{\frac {1}{n_{i}^{2}}}\right),\,n_{j}<n_{i}\,\!}
Many of the definitions below are also used in the thermodynamics of chemical reactions .
General basic quantities [ edit ]
Quantity (common name/s)
(Common) symbol/s
SI unit
Dimension
Number of molecules
N
1
1
Amount of substance
n
mol
N
Temperature
T
K
Θ
Heat Energy
Q , q
J
ML2 T−2
Latent heat
QL
J
ML2 T−2
General derived quantities [ edit ]
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI unit
Dimension
Thermodynamic beta , inverse temperature
β
β
=
1
/
k
B
T
{\displaystyle \beta =1/k_{\text{B}}T}
J−1
T2 M−1 L−2
Thermodynamic temperature
τ
τ
=
k
B
T
{\displaystyle \tau =k_{\text{B}}T}
τ
=
k
B
(
∂
U
/
∂
S
)
N
{\displaystyle \tau =k_{\text{B}}\left(\partial U/\partial S\right)_{N}}
1
/
τ
=
1
/
k
B
(
∂
S
/
∂
U
)
N
{\displaystyle 1/\tau =1/k_{\text{B}}\left(\partial S/\partial U\right)_{N}}
J
ML2 T−2
Entropy
S
S
=
−
k
B
∑
i
p
i
ln
p
i
{\displaystyle S=-k_{\text{B}}\sum _{i}p_{i}\ln p_{i}}
S
=
−
(
∂
F
/
∂
T
)
V
{\displaystyle S=-\left(\partial F/\partial T\right)_{V}}
S
=
−
(
∂
G
/
∂
T
)
N
,
P
{\displaystyle S=-\left(\partial G/\partial T\right)_{N,P}}
J⋅K−1
ML2 T−2 Θ−1
Pressure
P
P
=
−
(
∂
F
/
∂
V
)
T
,
N
{\displaystyle P=-\left(\partial F/\partial V\right)_{T,N}}
P
=
−
(
∂
U
/
∂
V
)
S
,
N
{\displaystyle P=-\left(\partial U/\partial V\right)_{S,N}}
Pa
ML−1 T−2
Internal Energy
U
U
=
∑
i
E
i
{\displaystyle U=\sum _{i}E_{i}}
J
ML2 T−2
Enthalpy
H
H
=
U
+
p
V
{\displaystyle H=U+pV}
J
ML2 T−2
Partition Function
Z
1
1
Gibbs free energy
G
G
=
H
−
T
S
{\displaystyle G=H-TS}
J
ML2 T−2
Chemical potential (of component i in a mixture)
μi
μ
i
=
(
∂
U
/
∂
N
i
)
N
j
≠
i
,
S
,
V
{\displaystyle \mu _{i}=\left(\partial U/\partial N_{i}\right)_{N_{j\neq i},S,V}}
μ
i
=
(
∂
F
/
∂
N
i
)
T
,
V
{\displaystyle \mu _{i}=\left(\partial F/\partial N_{i}\right)_{T,V}}
F
{\displaystyle F}
N
{\displaystyle N}
μ
i
{\displaystyle \mu _{i}}
μ
i
=
(
∂
G
/
∂
N
i
)
T
,
P
{\displaystyle \mu _{i}=\left(\partial G/\partial N_{i}\right)_{T,P}}
G
{\displaystyle G}
N
{\displaystyle N}
μ
i
{\displaystyle \mu _{i}}
μ
i
/
τ
=
−
1
/
k
B
(
∂
S
/
∂
N
i
)
U
,
V
{\displaystyle \mu _{i}/\tau =-1/k_{\text{B}}\left(\partial S/\partial N_{i}\right)_{U,V}}
J
ML2 T−2
Helmholtz free energy
A , F
F
=
U
−
T
S
{\displaystyle F=U-TS}
J
ML2 T−2
Landau potential , Landau free energy, Grand potential
Ω , ΦG
Ω
=
U
−
T
S
−
μ
N
{\displaystyle \Omega =U-TS-\mu N\ }
J
ML2 T−2
Massieu potential, Helmholtz free entropy
Φ
Φ
=
S
−
U
/
T
{\displaystyle \Phi =S-U/T}
J⋅K−1
ML2 T−2 Θ−1
Planck potential, Gibbs free entropy
Ξ
Ξ
=
Φ
−
p
V
/
T
{\displaystyle \Xi =\Phi -pV/T}
J⋅K−1
ML2 T−2 Θ−1
Thermal properties of matter [ edit ]
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI unit
Dimension
General heat/thermal capacity
C
C
=
∂
Q
/
∂
T
{\displaystyle C=\partial Q/\partial T}
J⋅K−1
ML2 T−2 Θ−1
Heat capacity (isobaric)
Cp
C
p
=
∂
H
/
∂
T
{\displaystyle C_{p}=\partial H/\partial T}
J⋅K−1
ML2 T−2 Θ−1
Specific heat capacity (isobaric)
Cmp
C
m
p
=
∂
2
Q
/
∂
m
∂
T
{\displaystyle C_{mp}=\partial ^{2}Q/\partial m\partial T}
J⋅kg−1 ⋅K−1
L2 T−2 Θ−1
Molar specific heat capacity (isobaric)
Cnp
C
n
p
=
∂
2
Q
/
∂
n
∂
T
{\displaystyle C_{np}=\partial ^{2}Q/\partial n\partial T}
J⋅K−1 ⋅mol−1
ML2 T−2 Θ−1 N−1
Heat capacity (isochoric/volumetric)
CV
C
V
=
∂
U
/
∂
T
{\displaystyle C_{V}=\partial U/\partial T}
J⋅K−1
ML2 T−2 Θ−1
Specific heat capacity (isochoric)
CmV
C
m
V
=
∂
2
Q
/
∂
m
∂
T
{\displaystyle C_{mV}=\partial ^{2}Q/\partial m\partial T}
J⋅kg−1 ⋅K−1
L2 T−2 Θ−1
Molar specific heat capacity (isochoric)
CnV
C
n
V
=
∂
2
Q
/
∂
n
∂
T
{\displaystyle C_{nV}=\partial ^{2}Q/\partial n\partial T}
J⋅K⋅−1 mol−1
ML2 T−2 Θ−1 N−1
Specific latent heat
L
L
=
∂
Q
/
∂
m
{\displaystyle L=\partial Q/\partial m}
J⋅kg−1
L2 T−2
Ratio of isobaric to isochoric heat capacity, heat capacity ratio , adiabatic index, Laplace coefficient
γ
γ
=
C
p
/
C
V
=
c
p
/
c
V
=
C
m
p
/
C
m
V
{\displaystyle \gamma =C_{p}/C_{V}=c_{p}/c_{V}=C_{mp}/C_{mV}}
1
1
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI unit
Dimension
Temperature gradient
No standard symbol
∇
T
{\displaystyle \nabla T}
K⋅m−1
ΘL−1
Thermal conduction rate, thermal current, thermal/heat flux , thermal power transfer
P
P
=
d
Q
/
d
t
{\displaystyle P=\mathrm {d} Q/\mathrm {d} t}
W
ML2 T−3
Thermal intensity
I
I
=
d
P
/
d
A
{\displaystyle I=\mathrm {d} P/\mathrm {d} A}
W⋅m−2
MT−3
Thermal/heat flux density (vector analogue of thermal intensity above)
q
Q
=
∬
q
⋅
d
S
d
t
{\displaystyle Q=\iint \mathbf {q} \cdot \mathrm {d} \mathbf {S} \mathrm {d} t}
W⋅m−2
MT−3
Thermodynamic processes [ edit ]
Physical situation
Equations
Isentropic process (adiabatic and reversible)
Q
=
0
,
Δ
U
=
−
W
{\displaystyle Q=0,\quad \Delta U=-W}
p
1
V
1
γ
=
p
2
V
2
γ
{\displaystyle p_{1}V_{1}^{\gamma }=p_{2}V_{2}^{\gamma }}
T
1
V
1
γ
−
1
=
T
2
V
2
γ
−
1
{\displaystyle T_{1}V_{1}^{\gamma -1}=T_{2}V_{2}^{\gamma -1}}
p
1
1
−
γ
T
1
γ
=
p
2
1
−
γ
T
2
γ
{\displaystyle p_{1}^{1-\gamma }T_{1}^{\gamma }=p_{2}^{1-\gamma }T_{2}^{\gamma }}
Isothermal process
Δ
U
=
0
,
W
=
Q
{\displaystyle \Delta U=0,\quad W=Q}
W
=
k
T
N
ln
(
V
2
/
V
1
)
{\displaystyle W=kTN\ln(V_{2}/V_{1})}
W
=
n
R
T
ln
(
V
2
/
V
1
)
{\displaystyle W=nRT\ln(V_{2}/V_{1})}
Isobaric process
p 1 = p 2 , p = constant
W
=
p
Δ
V
,
Q
=
Δ
U
+
p
δ
V
{\displaystyle W=p\Delta V,\quad Q=\Delta U+p\delta V}
Isochoric process
V 1 = V 2 , V = constant
W
=
0
,
Q
=
Δ
U
{\displaystyle W=0,\quad Q=\Delta U}
Free expansion
Δ
U
=
0
{\displaystyle \Delta U=0}
Work done by an expanding gas
Process
W
=
∫
V
1
V
2
p
d
V
{\displaystyle W=\int _{V_{1}}^{V_{2}}p\mathrm {d} V}
Net work done in cyclic processes
W
=
∮
c
y
c
l
e
p
d
V
=
∮
c
y
c
l
e
Δ
Q
{\displaystyle W=\oint _{\mathrm {cycle} }p\mathrm {d} V=\oint _{\mathrm {cycle} }\Delta Q}
S
=
k
B
ln
Ω
{\displaystyle S=k_{\mathrm {B} }\ln \Omega }
k B is the Boltzmann constant , and Ω denotes the volume of macrostate in the phase space or otherwise called thermodynamic probability.
d
S
=
δ
Q
T
{\displaystyle dS={\frac {\delta Q}{T}}}
Statistical physics [ edit ] Below are useful results from the Maxwell–Boltzmann distribution for an ideal gas, and the implications of the Entropy quantity. The distribution is valid for atoms or molecules constituting ideal gases.
Physical situation
Nomenclature
Equations
Maxwell–Boltzmann distribution
v = velocity of atom/molecule,m = mass of each molecule (all molecules are identical in kinetic theory),γ (p ) = Lorentz factor as function of momentum (see below)Ratio of thermal to rest mass-energy of each molecule:
θ
=
k
B
T
/
m
c
2
{\displaystyle \theta =k_{\text{B}}T/mc^{2}}
K 2 is the modified Bessel function of the second kind.
Non-relativistic speeds
P
(
v
)
=
4
π
(
m
2
π
k
B
T
)
3
/
2
v
2
e
−
m
v
2
/
2
k
B
T
{\displaystyle P\left(v\right)=4\pi \left({\frac {m}{2\pi k_{\text{B}}T}}\right)^{3/2}v^{2}e^{-mv^{2}/2k_{\text{B}}T}}
Relativistic speeds (Maxwell–Jüttner distribution)
f
(
p
)
=
1
4
π
m
3
c
3
θ
K
2
(
1
/
θ
)
e
−
γ
(
p
)
/
θ
{\displaystyle f(p)={\frac {1}{4\pi m^{3}c^{3}\theta K_{2}(1/\theta )}}e^{-\gamma (p)/\theta }}
Entropy Logarithm of the density of states
Pi = probability of system in microstate i Ω = total number of microstates
S
=
−
k
B
∑
i
P
i
ln
P
i
=
k
B
ln
Ω
{\displaystyle S=-k_{\text{B}}\sum _{i}P_{i}\ln P_{i}=k_{\mathrm {B} }\ln \Omega }
P
i
=
1
/
Ω
{\displaystyle P_{i}=1/\Omega }
Entropy change
Δ
S
=
∫
Q
1
Q
2
d
Q
T
{\displaystyle \Delta S=\int _{Q_{1}}^{Q_{2}}{\frac {\mathrm {d} Q}{T}}}
Δ
S
=
k
B
N
ln
V
2
V
1
+
N
C
V
ln
T
2
T
1
{\displaystyle \Delta S=k_{\text{B}}N\ln {\frac {V_{2}}{V_{1}}}+NC_{V}\ln {\frac {T_{2}}{T_{1}}}}
Entropic force
F
S
=
−
T
∇
S
{\displaystyle \mathbf {F} _{\mathrm {S} }=-T\nabla S}
Equipartition theorem
d f = degree of freedom
Average kinetic energy per degree of freedom
⟨
E
k
⟩
=
1
2
k
T
{\displaystyle \langle E_{\mathrm {k} }\rangle ={\frac {1}{2}}kT}
Internal energy
U
=
d
f
⟨
E
k
⟩
=
d
f
2
k
T
{\displaystyle U=d_{\text{f}}\langle E_{\mathrm {k} }\rangle ={\frac {d_{\text{f}}}{2}}kT}
Corollaries of the non-relativistic Maxwell–Boltzmann distribution are below.
Physical situation
Nomenclature
Equations
Mean speed
⟨
v
⟩
=
8
k
B
T
π
m
{\displaystyle \langle v\rangle ={\sqrt {\frac {8k_{\text{B}}T}{\pi m}}}}
Root mean square speed
v
r
m
s
=
⟨
v
2
⟩
=
3
k
B
T
m
{\displaystyle v_{\mathrm {rms} }={\sqrt {\langle v^{2}\rangle }}={\sqrt {\frac {3k_{\text{B}}T}{m}}}}
Modal speed
v
m
o
d
e
=
2
k
B
T
m
{\displaystyle v_{\mathrm {mode} }={\sqrt {\frac {2k_{\text{B}}T}{m}}}}
Mean free path
σ = effective cross-sectionn = volume density of number of target particlesℓ = mean free path
ℓ
=
1
/
2
n
σ
{\displaystyle \ell =1/{\sqrt {2}}n\sigma }
Quasi-static and reversible processes [ edit ] For quasi-static and reversible processes, the first law of thermodynamics is:
d
U
=
δ
Q
−
δ
W
{\displaystyle dU=\delta Q-\delta W}
where δQ is the heat supplied to the system and δW is the work done by the system.
Thermodynamic potentials [ edit ] The following energies are called the thermodynamic potentials .
Name
Symbol
Formula
Natural variables
Internal energy
U
{\displaystyle U}
∫
(
T
d
S
−
p
d
V
+
∑
i
μ
i
d
N
i
)
{\displaystyle \int \left(T\,\mathrm {d} S-p\,\mathrm {d} V+\sum _{i}\mu _{i}\mathrm {d} N_{i}\right)}
S
,
V
,
{
N
i
}
{\displaystyle S,V,\{N_{i}\}}
Helmholtz free energy
F
{\displaystyle F}
U
−
T
S
{\displaystyle U-TS}
T
,
V
,
{
N
i
}
{\displaystyle T,V,\{N_{i}\}}
Enthalpy
H
{\displaystyle H}
U
+
p
V
{\displaystyle U+pV}
S
,
p
,
{
N
i
}
{\displaystyle S,p,\{N_{i}\}}
Gibbs free energy
G
{\displaystyle G}
U
+
p
V
−
T
S
{\displaystyle U+pV-TS}
T
,
p
,
{
N
i
}
{\displaystyle T,p,\{N_{i}\}}
Landau potential, or
Ω
{\displaystyle \Omega }
Φ
G
{\displaystyle \Phi _{\text{G}}}
U
−
T
S
−
{\displaystyle U-TS-}
∑
i
{\displaystyle \sum _{i}\,}
μ
i
N
i
{\displaystyle \mu _{i}N_{i}}
T
,
V
,
{
μ
i
}
{\displaystyle T,V,\{\mu _{i}\}}
and the corresponding fundamental thermodynamic relations or "master equations"[ 4]
Potential
Differential
Internal energy
d
U
(
S
,
V
,
N
i
)
=
T
d
S
−
p
d
V
+
∑
i
μ
i
d
N
i
{\displaystyle dU\left(S,V,{N_{i}}\right)=TdS-pdV+\sum _{i}\mu _{i}dN_{i}}
Enthalpy
d
H
(
S
,
p
,
N
i
)
=
T
d
S
+
V
d
p
+
∑
i
μ
i
d
N
i
{\displaystyle dH\left(S,p,{N_{i}}\right)=TdS+Vdp+\sum _{i}\mu _{i}dN_{i}}
Helmholtz free energy
d
F
(
T
,
V
,
N
i
)
=
−
S
d
T
−
p
d
V
+
∑
i
μ
i
d
N
i
{\displaystyle dF\left(T,V,{N_{i}}\right)=-SdT-pdV+\sum _{i}\mu _{i}dN_{i}}
Gibbs free energy
d
G
(
T
,
p
,
N
i
)
=
−
S
d
T
+
V
d
p
+
∑
i
μ
i
d
N
i
{\displaystyle dG\left(T,p,{N_{i}}\right)=-SdT+Vdp+\sum _{i}\mu _{i}dN_{i}}
[ edit ] The four most common Maxwell's relations are:
Physical situation
Nomenclature
Equations
Thermodynamic potentials as functions of their natural variables
U
(
S
,
V
)
{\displaystyle U(S,V)\,}
Internal energy
H
(
S
,
P
)
{\displaystyle H(S,P)\,}
Enthalpy
F
(
T
,
V
)
{\displaystyle F(T,V)\,}
Helmholtz free energy
G
(
T
,
P
)
{\displaystyle G(T,P)\,}
Gibbs free energy
(
∂
T
∂
V
)
S
=
−
(
∂
P
∂
S
)
V
=
∂
2
U
∂
S
∂
V
{\displaystyle \left({\frac {\partial T}{\partial V}}\right)_{S}=-\left({\frac {\partial P}{\partial S}}\right)_{V}={\frac {\partial ^{2}U}{\partial S\partial V}}}
(
∂
T
∂
P
)
S
=
+
(
∂
V
∂
S
)
P
=
∂
2
H
∂
S
∂
P
{\displaystyle \left({\frac {\partial T}{\partial P}}\right)_{S}=+\left({\frac {\partial V}{\partial S}}\right)_{P}={\frac {\partial ^{2}H}{\partial S\partial P}}}
+
(
∂
S
∂
V
)
T
=
(
∂
P
∂
T
)
V
=
−
∂
2
F
∂
T
∂
V
{\displaystyle +\left({\frac {\partial S}{\partial V}}\right)_{T}=\left({\frac {\partial P}{\partial T}}\right)_{V}=-{\frac {\partial ^{2}F}{\partial T\partial V}}}
−
(
∂
S
∂
P
)
T
=
(
∂
V
∂
T
)
P
=
∂
2
G
∂
T
∂
P
{\displaystyle -\left({\frac {\partial S}{\partial P}}\right)_{T}=\left({\frac {\partial V}{\partial T}}\right)_{P}={\frac {\partial ^{2}G}{\partial T\partial P}}}
More relations include the following.
(
∂
S
∂
U
)
V
,
N
=
1
T
{\displaystyle \left({\partial S \over \partial U}\right)_{V,N}={1 \over T}}
(
∂
S
∂
V
)
N
,
U
=
p
T
{\displaystyle \left({\partial S \over \partial V}\right)_{N,U}={p \over T}}
(
∂
S
∂
N
)
V
,
U
=
−
μ
T
{\displaystyle \left({\partial S \over \partial N}\right)_{V,U}=-{\mu \over T}}
(
∂
T
∂
S
)
V
=
T
C
V
{\displaystyle \left({\partial T \over \partial S}\right)_{V}={T \over C_{V}}}
(
∂
T
∂
S
)
P
=
T
C
P
{\displaystyle \left({\partial T \over \partial S}\right)_{P}={T \over C_{P}}}
−
(
∂
p
∂
V
)
T
=
1
V
K
T
{\displaystyle -\left({\partial p \over \partial V}\right)_{T}={1 \over {VK_{T}}}}
Other differential equations are:
Name
H
U
G
Gibbs–Helmholtz equation
H
=
−
T
2
(
∂
(
G
/
T
)
∂
T
)
p
{\displaystyle H=-T^{2}\left({\frac {\partial \left(G/T\right)}{\partial T}}\right)_{p}}
U
=
−
T
2
(
∂
(
F
/
T
)
∂
T
)
V
{\displaystyle U=-T^{2}\left({\frac {\partial \left(F/T\right)}{\partial T}}\right)_{V}}
G
=
−
V
2
(
∂
(
F
/
V
)
∂
V
)
T
{\displaystyle G=-V^{2}\left({\frac {\partial \left(F/V\right)}{\partial V}}\right)_{T}}
(
∂
H
∂
p
)
T
=
V
−
T
(
∂
V
∂
T
)
P
{\displaystyle \left({\frac {\partial H}{\partial p}}\right)_{T}=V-T\left({\frac {\partial V}{\partial T}}\right)_{P}}
(
∂
U
∂
V
)
T
=
T
(
∂
P
∂
T
)
V
−
P
{\displaystyle \left({\frac {\partial U}{\partial V}}\right)_{T}=T\left({\frac {\partial P}{\partial T}}\right)_{V}-P}
U
=
N
k
B
T
2
(
∂
ln
Z
∂
T
)
V
{\displaystyle U=Nk_{\text{B}}T^{2}\left({\frac {\partial \ln Z}{\partial T}}\right)_{V}}
S
=
U
T
+
N
k
B
ln
Z
−
N
k
ln
N
+
N
k
{\displaystyle S={\frac {U}{T}}+Nk_{\text{B}}\ln Z-Nk\ln N+Nk}
where N is number of particles, h is that Planck constant , I is moment of inertia , and Z is the partition function , in various forms:
Degree of freedom
Partition function
Translation
Z
t
=
(
2
π
m
k
B
T
)
3
2
V
h
3
{\displaystyle Z_{t}={\frac {(2\pi mk_{\text{B}}T)^{\frac {3}{2}}V}{h^{3}}}}
Vibration
Z
v
=
1
1
−
e
−
h
ω
2
π
k
B
T
{\displaystyle Z_{v}={\frac {1}{1-e^{\frac {-h\omega }{2\pi k_{\text{B}}T}}}}}
Rotation
Z
r
=
2
I
k
B
T
σ
(
h
2
π
)
2
{\displaystyle Z_{r}={\frac {2Ik_{\text{B}}T}{\sigma ({\frac {h}{2\pi }})^{2}}}}
Thermal properties of matter [ edit ]
Coefficients
Equation
Joule-Thomson coefficient
μ
J
T
=
(
∂
T
∂
p
)
H
{\displaystyle \mu _{JT}=\left({\frac {\partial T}{\partial p}}\right)_{H}}
Compressibility (constant temperature)
K
T
=
−
1
V
(
∂
V
∂
p
)
T
,
N
{\displaystyle K_{T}=-{1 \over V}\left({\partial V \over \partial p}\right)_{T,N}}
Coefficient of thermal expansion (constant pressure)
α
p
=
1
V
(
∂
V
∂
T
)
p
{\displaystyle \alpha _{p}={\frac {1}{V}}\left({\frac {\partial V}{\partial T}}\right)_{p}}
Heat capacity (constant pressure)
C
p
=
(
∂
Q
r
e
v
∂
T
)
p
=
(
∂
U
∂
T
)
p
+
p
(
∂
V
∂
T
)
p
=
(
∂
H
∂
T
)
p
=
T
(
∂
S
∂
T
)
p
{\displaystyle C_{p}=\left({\partial Q_{rev} \over \partial T}\right)_{p}=\left({\partial U \over \partial T}\right)_{p}+p\left({\partial V \over \partial T}\right)_{p}=\left({\partial H \over \partial T}\right)_{p}=T\left({\partial S \over \partial T}\right)_{p}}
Heat capacity (constant volume)
C
V
=
(
∂
Q
r
e
v
∂
T
)
V
=
(
∂
U
∂
T
)
V
=
T
(
∂
S
∂
T
)
V
{\displaystyle C_{V}=\left({\partial Q_{rev} \over \partial T}\right)_{V}=\left({\partial U \over \partial T}\right)_{V}=T\left({\partial S \over \partial T}\right)_{V}}
Thermal efficiencies [ edit ]
Physical situation
Nomenclature
Equations
Thermodynamic engines
η = efficiencyW = work done by engineQ H = heat energy in higher temperature reservoirQ L = heat energy in lower temperature reservoirT H = temperature of higher temp. reservoirT L = temperature of lower temp. reservoir
Thermodynamic engine:
η
=
|
W
Q
H
|
{\displaystyle \eta =\left|{\frac {W}{Q_{\text{H}}}}\right|}
Carnot engine efficiency:
η
c
=
1
−
|
Q
L
Q
H
|
=
1
−
T
L
T
H
{\displaystyle \eta _{\text{c}}=1-\left|{\frac {Q_{\text{L}}}{Q_{\text{H}}}}\right|=1-{\frac {T_{\text{L}}}{T_{\text{H}}}}}
Refrigeration
K = coefficient of refrigeration performance
Refrigeration performance
K
=
|
Q
L
W
|
{\displaystyle K=\left|{\frac {Q_{\text{L}}}{W}}\right|}
Carnot refrigeration performance
K
C
=
|
Q
L
|
|
Q
H
|
−
|
Q
L
|
=
T
L
T
H
−
T
L
{\displaystyle K_{\text{C}}={\frac {|Q_{\text{L}}|}{|Q_{\text{H}}|-|Q_{\text{L}}|}}={\frac {T_{\text{L}}}{T_{\text{H}}-T_{\text{L}}}}}
Classical mechanics [ edit ] Classical mechanics [ edit ]
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI units
Dimension
Linear, surface, volumetric mass density
λ or μ (especially in acoustics , see below) for Linear, σ for surface, ρ for volume.
m
=
∫
λ
d
ℓ
{\displaystyle m=\int \lambda \,\mathrm {d} \ell }
m
=
∬
σ
d
S
{\displaystyle m=\iint \sigma \,\mathrm {d} S}
m
=
∭
ρ
d
V
{\displaystyle m=\iiint \rho \,\mathrm {d} V}
kg m−n , n = 1, 2, 3
M L−n
Moment of mass[ 5]
m (No common symbol)
Point mass:
m
=
r
m
{\displaystyle \mathbf {m} =\mathbf {r} m}
Discrete masses about an axis
x
i
{\displaystyle x_{i}}
m
=
∑
i
=
1
N
r
i
m
i
{\displaystyle \mathbf {m} =\sum _{i=1}^{N}\mathbf {r} _{i}m_{i}}
Continuum of mass about an axis
x
i
{\displaystyle x_{i}}
m
=
∫
ρ
(
r
)
x
i
d
r
{\displaystyle \mathbf {m} =\int \rho \left(\mathbf {r} \right)x_{i}\mathrm {d} \mathbf {r} }
kg m
M L
Center of mass
r com
(Symbols vary)
i -th moment of mass
m
i
=
r
i
m
i
{\displaystyle \mathbf {m} _{i}=\mathbf {r} _{i}m_{i}}
r
c
o
m
=
1
M
∑
i
r
i
m
i
=
1
M
∑
i
m
i
{\displaystyle \mathbf {r} _{\mathrm {com} }={\frac {1}{M}}\sum _{i}\mathbf {r} _{i}m_{i}={\frac {1}{M}}\sum _{i}\mathbf {m} _{i}}
Mass continuum:
r
c
o
m
=
1
M
∫
d
m
=
1
M
∫
r
d
m
=
1
M
∫
r
ρ
d
V
{\displaystyle \mathbf {r} _{\mathrm {com} }={\frac {1}{M}}\int \mathrm {d} \mathbf {m} ={\frac {1}{M}}\int \mathbf {r} \,\mathrm {d} m={\frac {1}{M}}\int \mathbf {r} \rho \,\mathrm {d} V}
m
L
2-Body reduced mass
m 12 , μ Pair of masses = m 1 and m 2
μ
=
m
1
m
2
m
1
+
m
2
{\displaystyle \mu ={\frac {m_{1}m_{2}}{m_{1}+m_{2}}}}
kg
M
Moment of inertia (MOI)
I
Discrete Masses:
I
=
∑
i
m
i
⋅
r
i
=
∑
i
|
r
i
|
2
m
{\displaystyle I=\sum _{i}\mathbf {m} _{i}\cdot \mathbf {r} _{i}=\sum _{i}\left|\mathbf {r} _{i}\right|^{2}m}
Mass continuum:
I
=
∫
|
r
|
2
d
m
=
∫
r
⋅
d
m
=
∫
|
r
|
2
ρ
d
V
{\displaystyle I=\int \left|\mathbf {r} \right|^{2}\mathrm {d} m=\int \mathbf {r} \cdot \mathrm {d} \mathbf {m} =\int \left|\mathbf {r} \right|^{2}\rho \,\mathrm {d} V}
kg m2
M L2
Derived kinematic quantities [ edit ] Kinematic quantities of a classical particle: mass m , position r , velocity v , acceleration a .
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI units
Dimension
Velocity
v
v
=
d
r
d
t
{\displaystyle \mathbf {v} ={\frac {\mathrm {d} \mathbf {r} }{\mathrm {d} t}}}
m s−1
L T−1
Acceleration
a
a
=
d
v
d
t
=
d
2
r
d
t
2
{\displaystyle \mathbf {a} ={\frac {\mathrm {d} \mathbf {v} }{\mathrm {d} t}}={\frac {\mathrm {d} ^{2}\mathbf {r} }{\mathrm {d} t^{2}}}}
m s−2
L T−2
Jerk
j
j
=
d
a
d
t
=
d
3
r
d
t
3
{\displaystyle \mathbf {j} ={\frac {\mathrm {d} \mathbf {a} }{\mathrm {d} t}}={\frac {\mathrm {d} ^{3}\mathbf {r} }{\mathrm {d} t^{3}}}}
m s−3
L T−3
Jounce
s
s
=
d
j
d
t
=
d
4
r
d
t
4
{\displaystyle \mathbf {s} ={\frac {\mathrm {d} \mathbf {j} }{\mathrm {d} t}}={\frac {\mathrm {d} ^{4}\mathbf {r} }{\mathrm {d} t^{4}}}}
m s−4
L T−4
Angular velocity
ω
ω
=
n
^
d
θ
d
t
{\displaystyle {\boldsymbol {\omega }}=\mathbf {\hat {n}} {\frac {\mathrm {d} \theta }{\mathrm {d} t}}}
rad s−1
T−1
Angular Acceleration
α
α
=
d
ω
d
t
=
n
^
d
2
θ
d
t
2
{\displaystyle {\boldsymbol {\alpha }}={\frac {\mathrm {d} {\boldsymbol {\omega }}}{\mathrm {d} t}}=\mathbf {\hat {n}} {\frac {\mathrm {d} ^{2}\theta }{\mathrm {d} t^{2}}}}
rad s−2
T−2
Angular jerk
ζ
ζ
=
d
α
d
t
=
n
^
d
3
θ
d
t
3
{\displaystyle {\boldsymbol {\zeta }}={\frac {\mathrm {d} {\boldsymbol {\alpha }}}{\mathrm {d} t}}=\mathbf {\hat {n}} {\frac {\mathrm {d} ^{3}\theta }{\mathrm {d} t^{3}}}}
rad s−3
T−3
Derived dynamic quantities [ edit ] Angular momenta of a classical object.Left: intrinsic "spin" angular momentum S is really orbital angular momentum of the object at every point, right: extrinsic orbital angular momentum L about an axis, top: the moment of inertia tensor I and angular velocity ω (L is not always parallel to ω )[ 6] bottom: momentum p and its radial position r from the axis. The total angular momentum (spin + orbital) is J . General energy definitions [ edit ]
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI units
Dimension
Mechanical work due to a Resultant Force
W
W
=
∫
C
F
⋅
d
r
{\displaystyle W=\int _{C}\mathbf {F} \cdot \mathrm {d} \mathbf {r} }
J = N m = kg m2 s−2
M L2 T−2
Work done ON mechanical system, Work done BY
W ON , W BY
Δ
W
O
N
=
−
Δ
W
B
Y
{\displaystyle \Delta W_{\mathrm {ON} }=-\Delta W_{\mathrm {BY} }}
J = N m = kg m2 s−2
M L2 T−2
Potential energy
φ , Φ, U , V , Ep
Δ
W
=
−
Δ
V
{\displaystyle \Delta W=-\Delta V}
J = N m = kg m2 s−2
M L2 T−2
Mechanical power
P
P
=
d
E
d
t
{\displaystyle P={\frac {\mathrm {d} E}{\mathrm {d} t}}}
W = J s−1
M L2 T−3
Every conservative force has a potential energy . By following two principles one can consistently assign a non-relative value to U :
Wherever the force is zero, its potential energy is defined to be zero as well.
Whenever the force does work, potential energy is lost. Generalized mechanics [ edit ]
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI units
Dimension
Generalized coordinates
q, Q
varies with choice
varies with choice
Generalized velocities
q
˙
,
Q
˙
{\displaystyle {\dot {q}},{\dot {Q}}}
q
˙
≡
d
q
/
d
t
{\displaystyle {\dot {q}}\equiv \mathrm {d} q/\mathrm {d} t}
varies with choice
varies with choice
Generalized momenta
p, P
p
=
∂
L
/
∂
q
˙
{\displaystyle p=\partial L/\partial {\dot {q}}}
varies with choice
varies with choice
Lagrangian
L
L
(
q
,
q
˙
,
t
)
=
T
(
q
˙
)
−
V
(
q
,
q
˙
,
t
)
{\displaystyle L(\mathbf {q} ,\mathbf {\dot {q}} ,t)=T(\mathbf {\dot {q}} )-V(\mathbf {q} ,\mathbf {\dot {q}} ,t)}
q
=
q
(
t
)
{\displaystyle \mathbf {q} =\mathbf {q} (t)}
p = p (t ) are vectors of the generalized coords and momenta, as functions of time
J
M L2 T−2
Hamiltonian
H
H
(
p
,
q
,
t
)
=
p
⋅
q
˙
−
L
(
q
,
q
˙
,
t
)
{\displaystyle H(\mathbf {p} ,\mathbf {q} ,t)=\mathbf {p} \cdot \mathbf {\dot {q}} -L(\mathbf {q} ,\mathbf {\dot {q}} ,t)}
J
M L2 T−2
Action , Hamilton's principal function
S ,
S
{\displaystyle \scriptstyle {\mathcal {S}}}
S
=
∫
t
1
t
2
L
(
q
,
q
˙
,
t
)
d
t
{\displaystyle {\mathcal {S}}=\int _{t_{1}}^{t_{2}}L(\mathbf {q} ,\mathbf {\dot {q}} ,t)\mathrm {d} t}
J s
M L2 T−1
In the following rotational definitions, the angle can be any angle about the specified axis of rotation. It is customary to use θ , but this does not have to be the polar angle used in polar coordinate systems. The unit axial vector
n
^
=
e
^
r
×
e
^
θ
{\displaystyle \mathbf {\hat {n}} =\mathbf {\hat {e}} _{r}\times \mathbf {\hat {e}} _{\theta }}
defines the axis of rotation,
e
^
r
{\displaystyle \scriptstyle \mathbf {\hat {e}} _{r}}
r
e
^
θ
{\displaystyle \scriptstyle \mathbf {\hat {e}} _{\theta }}
Translation
Rotation
Velocity
Average:
v
a
v
e
r
a
g
e
=
Δ
r
Δ
t
{\displaystyle \mathbf {v} _{\mathrm {average} }={\Delta \mathbf {r} \over \Delta t}}
v
=
d
r
d
t
{\displaystyle \mathbf {v} ={d\mathbf {r} \over dt}}
Angular velocity
ω
=
n
^
d
θ
d
t
{\displaystyle {\boldsymbol {\omega }}=\mathbf {\hat {n}} {\frac {{\rm {d}}\theta }{{\rm {d}}t}}}
rigid body :
v
=
ω
×
r
{\displaystyle \mathbf {v} ={\boldsymbol {\omega }}\times \mathbf {r} }
Acceleration
Average:
a
a
v
e
r
a
g
e
=
Δ
v
Δ
t
{\displaystyle \mathbf {a} _{\mathrm {average} }={\frac {\Delta \mathbf {v} }{\Delta t}}}
Instantaneous:
a
=
d
v
d
t
=
d
2
r
d
t
2
{\displaystyle \mathbf {a} ={\frac {d\mathbf {v} }{dt}}={\frac {d^{2}\mathbf {r} }{dt^{2}}}}
Angular acceleration
α
=
d
ω
d
t
=
n
^
d
2
θ
d
t
2
{\displaystyle {\boldsymbol {\alpha }}={\frac {{\rm {d}}{\boldsymbol {\omega }}}{{\rm {d}}t}}=\mathbf {\hat {n}} {\frac {{\rm {d}}^{2}\theta }{{\rm {d}}t^{2}}}}
Rotating rigid body:
a
=
α
×
r
+
ω
×
v
{\displaystyle \mathbf {a} ={\boldsymbol {\alpha }}\times \mathbf {r} +{\boldsymbol {\omega }}\times \mathbf {v} }
Jerk
Average:
j
a
v
e
r
a
g
e
=
Δ
a
Δ
t
{\displaystyle \mathbf {j} _{\mathrm {average} }={\frac {\Delta \mathbf {a} }{\Delta t}}}
Instantaneous:
j
=
d
a
d
t
=
d
2
v
d
t
2
=
d
3
r
d
t
3
{\displaystyle \mathbf {j} ={\frac {d\mathbf {a} }{dt}}={\frac {d^{2}\mathbf {v} }{dt^{2}}}={\frac {d^{3}\mathbf {r} }{dt^{3}}}}
Angular jerk
ζ
=
d
α
d
t
=
n
^
d
2
ω
d
t
2
=
n
^
d
3
θ
d
t
3
{\displaystyle {\boldsymbol {\zeta }}={\frac {{\rm {d}}{\boldsymbol {\alpha }}}{{\rm {d}}t}}=\mathbf {\hat {n}} {\frac {{\rm {d}}^{2}\omega }{{\rm {d}}t^{2}}}=\mathbf {\hat {n}} {\frac {{\rm {d}}^{3}\theta }{{\rm {d}}t^{3}}}}
Rotating rigid body:
j
=
ζ
×
r
+
α
×
a
{\displaystyle \mathbf {j} ={\boldsymbol {\zeta }}\times \mathbf {r} +{\boldsymbol {\alpha }}\times \mathbf {a} }
Translation
Rotation
Momentum
Momentum is the "amount of translation"
p
=
m
v
{\displaystyle \mathbf {p} =m\mathbf {v} }
For a rotating rigid body:
p
=
ω
×
m
{\displaystyle \mathbf {p} ={\boldsymbol {\omega }}\times \mathbf {m} }
Angular momentum
Angular momentum is the "amount of rotation":
L
=
r
×
p
=
I
⋅
ω
{\displaystyle \mathbf {L} =\mathbf {r} \times \mathbf {p} =\mathbf {I} \cdot {\boldsymbol {\omega }}}
and the cross-product is a pseudovector i.e. if r and p are reversed in direction (negative), L is not.
In general I is an order-2 tensor , see above for its components. The dot · indicates tensor contraction .
Force and Newton's 2nd law
Resultant force acts on a system at the center of mass, equal to the rate of change of momentum:
F
=
d
p
d
t
=
d
(
m
v
)
d
t
=
m
a
+
v
d
m
d
t
{\displaystyle {\begin{aligned}\mathbf {F} &={\frac {d\mathbf {p} }{dt}}={\frac {d(m\mathbf {v} )}{dt}}\\&=m\mathbf {a} +\mathbf {v} {\frac {{\rm {d}}m}{{\rm {d}}t}}\\\end{aligned}}}
For a number of particles, the equation of motion for one particle i is:[ 7]
d
p
i
d
t
=
F
E
+
∑
i
≠
j
F
i
j
{\displaystyle {\frac {\mathrm {d} \mathbf {p} _{i}}{\mathrm {d} t}}=\mathbf {F} _{E}+\sum _{i\neq j}\mathbf {F} _{ij}}
where p i i , F ij on i by j , and F E i does not exert a force on itself.
Torque
Torque τ is also called moment of a force, because it is the rotational analogue to force:[ 8]
τ
=
d
L
d
t
=
r
×
F
=
d
(
I
⋅
ω
)
d
t
{\displaystyle {\boldsymbol {\tau }}={\frac {{\rm {d}}\mathbf {L} }{{\rm {d}}t}}=\mathbf {r} \times \mathbf {F} ={\frac {{\rm {d}}(\mathbf {I} \cdot {\boldsymbol {\omega }})}{{\rm {d}}t}}}
For rigid bodies, Newton's 2nd law for rotation takes the same form as for translation:
τ
=
d
L
d
t
=
d
(
I
⋅
ω
)
d
t
=
d
I
d
t
⋅
ω
+
I
⋅
α
{\displaystyle {\begin{aligned}{\boldsymbol {\tau }}&={\frac {{\rm {d}}\mathbf {L} }{{\rm {d}}t}}={\frac {{\rm {d}}(\mathbf {I} \cdot {\boldsymbol {\omega }})}{{\rm {d}}t}}\\&={\frac {{\rm {d}}\mathbf {I} }{{\rm {d}}t}}\cdot {\boldsymbol {\omega }}+\mathbf {I} \cdot {\boldsymbol {\alpha }}\\\end{aligned}}}
Likewise, for a number of particles, the equation of motion for one particle i is:[ 9]
d
L
i
d
t
=
τ
E
+
∑
i
≠
j
τ
i
j
{\displaystyle {\frac {\mathrm {d} \mathbf {L} _{i}}{\mathrm {d} t}}={\boldsymbol {\tau }}_{E}+\sum _{i\neq j}{\boldsymbol {\tau }}_{ij}}
Yank
Yank is rate of change of force:
Y
=
d
F
d
t
=
d
2
p
d
t
2
=
d
2
(
m
v
)
d
t
2
=
m
j
+
2
a
d
m
d
t
+
v
d
2
m
d
t
2
{\displaystyle {\begin{aligned}\mathbf {Y} &={\frac {d\mathbf {F} }{dt}}={\frac {d^{2}\mathbf {p} }{dt^{2}}}={\frac {d^{2}(m\mathbf {v} )}{dt^{2}}}\\[1ex]&=m\mathbf {j} +\mathbf {2a} {\frac {{\rm {d}}m}{{\rm {d}}t}}+\mathbf {v} {\frac {{\rm {d^{2}}}m}{{\rm {d}}t^{2}}}\end{aligned}}}
For constant mass, it becomes;
Y
=
m
j
{\displaystyle \mathbf {Y} =m\mathbf {j} }
Rotatum
Rotatum Ρ is also called moment of a Yank, because it is the rotational analogue to yank:
P
=
d
τ
d
t
=
r
×
Y
=
d
(
I
⋅
α
)
d
t
{\displaystyle {\boldsymbol {\mathrm {P} }}={\frac {{\rm {d}}{\boldsymbol {\tau }}}{{\rm {d}}t}}=\mathbf {r} \times \mathbf {Y} ={\frac {{\rm {d}}(\mathbf {I} \cdot {\boldsymbol {\alpha }})}{{\rm {d}}t}}}
Impulse
Impulse is the change in momentum:
Δ
p
=
∫
F
d
t
{\displaystyle \Delta \mathbf {p} =\int \mathbf {F} \,dt}
For constant force F :
Δ
p
=
F
Δ
t
{\displaystyle \Delta \mathbf {p} =\mathbf {F} \Delta t}
Twirl/angular impulse is the change in angular momentum:
Δ
L
=
∫
τ
d
t
{\displaystyle \Delta \mathbf {L} =\int {\boldsymbol {\tau }}\,dt}
For constant torque τ :
Δ
L
=
τ
Δ
t
{\displaystyle \Delta \mathbf {L} ={\boldsymbol {\tau }}\Delta t}
The precession angular speed of a spinning top is given by:
Ω
=
w
r
I
ω
{\displaystyle {\boldsymbol {\Omega }}={\frac {wr}{I{\boldsymbol {\omega }}}}}
where w is the weight of the spinning flywheel.
The mechanical work done by an external agent on a system is equal to the change in kinetic energy of the system:
The work done W by an external agent which exerts a force F (at r ) and torque τ on an object along a curved path C is:
W
=
Δ
T
=
∫
C
(
F
⋅
d
r
+
τ
⋅
n
d
θ
)
{\displaystyle W=\Delta T=\int _{C}\left(\mathbf {F} \cdot \mathrm {d} \mathbf {r} +{\boldsymbol {\tau }}\cdot \mathbf {n} \,{\mathrm {d} \theta }\right)}
where θ is the angle of rotation about an axis defined by a unit vector n .
The change in kinetic energy for an object initially traveling at speed
v
0
{\displaystyle v_{0}}
v
{\displaystyle v}
Δ
E
k
=
W
=
1
2
m
(
v
2
−
v
0
2
)
{\displaystyle \Delta E_{k}=W={\frac {1}{2}}m(v^{2}-{v_{0}}^{2})}
Elastic potential energy [ edit ] For a stretched spring fixed at one end obeying Hooke's law , the elastic potential energy is
Δ
E
p
=
1
2
k
(
r
2
−
r
1
)
2
{\displaystyle \Delta E_{p}={\frac {1}{2}}k(r_{2}-r_{1})^{2}}
where r 2 and r 1 are collinear coordinates of the free end of the spring, in the direction of the extension/compression, and k is the spring constant.
[ edit ] Euler also worked out analogous laws of motion to those of Newton, see Euler's laws of motion . These extend the scope of Newton's laws to rigid bodies, but are essentially the same as above. A new equation Euler formulated is:[ 10]
I
⋅
α
+
ω
×
(
I
⋅
ω
)
=
τ
{\displaystyle \mathbf {I} \cdot {\boldsymbol {\alpha }}+{\boldsymbol {\omega }}\times \left(\mathbf {I} \cdot {\boldsymbol {\omega }}\right)={\boldsymbol {\tau }}}
where I is the moment of inertia tensor .
General planar motion [ edit ] The previous equations for planar motion can be used here: corollaries of momentum, angular momentum etc. can immediately follow by applying the above definitions. For any object moving in any path in a plane,
r
=
r
(
t
)
=
r
r
^
{\displaystyle \mathbf {r} =\mathbf {r} (t)=r{\hat {\mathbf {r} }}}
the following general results apply to the particle.
Kinematics
Dynamics
Position
r
=
r
(
r
,
θ
,
t
)
=
r
r
^
{\displaystyle \mathbf {r} =\mathbf {r} \left(r,\theta ,t\right)=r{\hat {\mathbf {r} }}}
Velocity
v
=
r
^
d
r
d
t
+
r
ω
θ
^
{\displaystyle \mathbf {v} ={\hat {\mathbf {r} }}{\frac {\mathrm {d} r}{\mathrm {d} t}}+r\omega {\hat {\mathbf {\theta } }}}
Momentum
p
=
m
(
r
^
d
r
d
t
+
r
ω
θ
^
)
{\displaystyle \mathbf {p} =m\left({\hat {\mathbf {r} }}{\frac {\mathrm {d} r}{\mathrm {d} t}}+r\omega {\hat {\mathbf {\theta } }}\right)}
Angular momenta
L
=
m
r
×
(
r
^
d
r
d
t
+
r
ω
θ
^
)
{\displaystyle \mathbf {L} =m\mathbf {r} \times \left({\hat {\mathbf {r} }}{\frac {\mathrm {d} r}{\mathrm {d} t}}+r\omega {\hat {\mathbf {\theta } }}\right)}
Acceleration
a
=
(
d
2
r
d
t
2
−
r
ω
2
)
r
^
+
(
r
α
+
2
ω
d
r
d
t
)
θ
^
{\displaystyle \mathbf {a} =\left({\frac {\mathrm {d} ^{2}r}{\mathrm {d} t^{2}}}-r\omega ^{2}\right){\hat {\mathbf {r} }}+\left(r\alpha +2\omega {\frac {\mathrm {d} r}{{\rm {d}}t}}\right){\hat {\mathbf {\theta } }}}
The centripetal force is
F
⊥
=
−
m
ω
2
R
r
^
=
−
ω
2
m
{\displaystyle \mathbf {F} _{\bot }=-m\omega ^{2}R{\hat {\mathbf {r} }}=-\omega ^{2}\mathbf {m} }
where again m is the mass moment, and the Coriolis force is
F
c
=
2
ω
m
d
r
d
t
θ
^
=
2
ω
m
v
θ
^
{\displaystyle \mathbf {F} _{c}=2\omega m{\frac {{\rm {d}}r}{{\rm {d}}t}}{\hat {\mathbf {\theta } }}=2\omega mv{\hat {\mathbf {\theta } }}}
The Coriolis acceleration and force can also be written:
F
c
=
m
a
c
=
−
2
m
ω
×
v
{\displaystyle \mathbf {F} _{c}=m\mathbf {a} _{c}=-2m{\boldsymbol {\omega \times v}}}
Central force motion [ edit ] For a massive body moving in a central potential due to another object, which depends only on the radial separation between the centers of masses of the two objects, the equation of motion is:
d
2
d
θ
2
(
1
r
)
+
1
r
=
−
μ
r
2
l
2
F
(
r
)
{\displaystyle {\frac {d^{2}}{d\theta ^{2}}}\left({\frac {1}{\mathbf {r} }}\right)+{\frac {1}{\mathbf {r} }}=-{\frac {\mu \mathbf {r} ^{2}}{\mathbf {l} ^{2}}}\mathbf {F} (\mathbf {r} )}
[ edit ] These equations can be used only when acceleration is constant. If acceleration is not constant then the general calculus equations above must be used, found by integrating the definitions of position, velocity and acceleration (see above).
Linear motion
Angular motion
v
−
v
0
=
a
t
{\displaystyle \mathbf {v-v_{0}} =\mathbf {a} t}
ω
−
ω
0
=
α
t
{\displaystyle {\boldsymbol {\omega -\omega _{0}}}={\boldsymbol {\alpha }}t}
x
−
x
0
=
1
2
(
v
0
+
v
)
t
{\displaystyle \mathbf {x-x_{0}} ={\tfrac {1}{2}}(\mathbf {v_{0}+v} )t}
θ
−
θ
0
=
1
2
(
ω
0
+
ω
)
t
{\displaystyle {\boldsymbol {\theta -\theta _{0}}}={\tfrac {1}{2}}({\boldsymbol {\omega _{0}+\omega }})t}
x
−
x
0
=
v
0
t
+
1
2
a
t
2
{\displaystyle \mathbf {x-x_{0}} =\mathbf {v} _{0}t+{\tfrac {1}{2}}\mathbf {a} t^{2}}
θ
−
θ
0
=
ω
0
t
+
1
2
α
t
2
{\displaystyle {\boldsymbol {\theta -\theta _{0}}}={\boldsymbol {\omega }}_{0}t+{\tfrac {1}{2}}{\boldsymbol {\alpha }}t^{2}}
x
n
t
h
=
v
0
+
a
(
n
−
1
2
)
{\displaystyle \mathbf {x} _{n^{th}}=\mathbf {v} _{0}+\mathbf {a} (n-{\tfrac {1}{2}})}
θ
n
t
h
=
ω
0
+
α
(
n
−
1
2
)
{\displaystyle {\boldsymbol {\theta }}_{n^{th}}={\boldsymbol {\omega }}_{0}+{\boldsymbol {\alpha }}(n-{\tfrac {1}{2}})}
v
2
−
v
0
2
=
2
a
(
x
−
x
0
)
{\displaystyle v^{2}-v_{0}^{2}=2\mathbf {a(x-x_{0})} }
ω
2
−
ω
0
2
=
2
α
(
θ
−
θ
0
)
{\displaystyle \omega ^{2}-\omega _{0}^{2}=2{\boldsymbol {\alpha (\theta -\theta _{0})}}}
For classical (Galileo-Newtonian) mechanics, the transformation law from one inertial or accelerating (including rotation) frame (reference frame traveling at constant velocity - including zero) to another is the Galilean transform.
Unprimed quantities refer to position, velocity and acceleration in one frame F; primed quantities refer to position, velocity and acceleration in another frame F' moving at translational velocity V or angular velocity Ω relative to F. Conversely F moves at velocity (—V or —Ω ) relative to F'. The situation is similar for relative accelerations.
Motion of entities
Inertial frames
Accelerating frames
Translation
V = Constant relative velocity between two inertial frames F and F'.
A = (Variable) relative acceleration between two accelerating frames F and F'.
Relative position
r
′
=
r
+
V
t
{\displaystyle \mathbf {r} '=\mathbf {r} +\mathbf {V} t}
Relative velocity
v
′
=
v
+
V
{\displaystyle \mathbf {v} '=\mathbf {v} +\mathbf {V} }
Equivalent accelerations
a
′
=
a
{\displaystyle \mathbf {a} '=\mathbf {a} }
Relative accelerations
a
′
=
a
+
A
{\displaystyle \mathbf {a} '=\mathbf {a} +\mathbf {A} }
Apparent/fictitious forces
F
′
=
F
−
F
a
p
p
{\displaystyle \mathbf {F} '=\mathbf {F} -\mathbf {F} _{\mathrm {app} }}
Rotation
Ω = Constant relative angular velocity between two frames F and F'.
Λ = (Variable) relative angular acceleration between two accelerating frames F and F'.
Relative angular position
θ
′
=
θ
+
Ω
t
{\displaystyle \theta '=\theta +\Omega t}
ω
′
=
ω
+
Ω
{\displaystyle {\boldsymbol {\omega }}'={\boldsymbol {\omega }}+{\boldsymbol {\Omega }}}
Equivalent accelerations
α
′
=
α
{\displaystyle {\boldsymbol {\alpha }}'={\boldsymbol {\alpha }}}
Relative accelerations
α
′
=
α
+
Λ
{\displaystyle {\boldsymbol {\alpha }}'={\boldsymbol {\alpha }}+{\boldsymbol {\Lambda }}}
Apparent/fictitious torques
τ
′
=
τ
−
τ
a
p
p
{\displaystyle {\boldsymbol {\tau }}'={\boldsymbol {\tau }}-{\boldsymbol {\tau }}_{\mathrm {app} }}
Transformation of any vector T to a rotating frame
d
T
′
d
t
=
d
T
d
t
−
Ω
×
T
{\displaystyle {\frac {{\rm {d}}\mathbf {T} '}{{\rm {d}}t}}={\frac {{\rm {d}}\mathbf {T} }{{\rm {d}}t}}-{\boldsymbol {\Omega }}\times \mathbf {T} }
Mechanical oscillators [ edit ] SHM, DHM, SHO, and DHO refer to simple harmonic motion, damped harmonic motion, simple harmonic oscillator and damped harmonic oscillator respectively.
General fundamental quantities [ edit ] A wave can be longitudinal where the oscillations are parallel (or antiparallel) to the propagation direction, or transverse where the oscillations are perpendicular to the propagation direction. These oscillations are characterized by a periodically time-varying displacement in the parallel or perpendicular direction, and so the instantaneous velocity and acceleration are also periodic and time varying in these directions. (the apparent motion of the wave due to the successive oscillations of particles or fields about their equilibrium positions) propagates at the phase and group velocities parallel or antiparallel to the propagation direction, which is common to longitudinal and transverse waves. Below oscillatory displacement, velocity and acceleration refer to the kinematics in the oscillating directions of the wave - transverse or longitudinal (mathematical description is identical), the group and phase velocities are separate.
Quantity (common name/s)
(Common) symbol/s
SI units
Dimension
Number of wave cycles
N
dimensionless
dimensionless
(Oscillatory) displacement
Symbol of any quantity which varies periodically, such as h , x , y (mechanical waves), x , s , η (longitudinal waves) I , V , E , B , H , D (electromagnetism), u , U (luminal waves), ψ , Ψ , Φ (quantum mechanics). Most general purposes use y , ψ , Ψ . For generality here, A is used and can be replaced by any other symbol, since others have specific, common uses.
A
=
A
e
^
∥
{\displaystyle \mathbf {A} =A\mathbf {\hat {e}} _{\parallel }\,\!}
A
=
A
e
^
⊥
{\displaystyle \mathbf {A} =A\mathbf {\hat {e}} _{\bot }\,\!}
m
[L]
(Oscillatory) displacement amplitude
Any quantity symbol typically subscripted with 0, m or max, or the capitalized letter (if displacement was in lower case). Here for generality A0 is used and can be replaced.
m
[L]
(Oscillatory) velocity amplitude
V , v 0 , vm . Here v 0 is used.
m s−1
[L][T]−1
(Oscillatory) acceleration amplitude
A , a 0 , am . Here a 0 is used.
m s−2
[L][T]−2
Spatial position
Position of a point in space, not necessarily a point on the wave profile or any line of propagation
d , r
m
[L]
Wave profile displacement
Along propagation direction, distance travelled (path length) by one wave from the source point r 0 to any point in space d (for longitudinal or transverse waves)
L , d , r
r
≡
r
e
^
∥
≡
d
−
r
0
{\displaystyle \mathbf {r} \equiv r\mathbf {\hat {e}} _{\parallel }\equiv \mathbf {d} -\mathbf {r} _{0}\,\!}
m
[L]
Phase angle
δ, ε, φ
rad
dimensionless
General derived quantities [ edit ]
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI units
Dimension
Wavelength
λ
General definition (allows for FM ):
λ
=
d
r
/
d
N
{\displaystyle \lambda =\mathrm {d} r/\mathrm {d} N\,\!}
For non-FM waves this reduces to:
λ
=
Δ
r
/
Δ
N
{\displaystyle \lambda =\Delta r/\Delta N\,\!}
m
[L]
Wavenumber, k -vector, Wave vector
k , σ
Two definitions are in use:
k
=
(
2
π
/
λ
)
e
^
∠
{\displaystyle \mathbf {k} =\left(2\pi /\lambda \right)\mathbf {\hat {e}} _{\angle }\,\!}
k
=
(
1
/
λ
)
e
^
∠
{\displaystyle \mathbf {k} =\left(1/\lambda \right)\mathbf {\hat {e}} _{\angle }\,\!}
m−1
[L]−1
Frequency
f, ν
General definition (allows for FM ):
f
=
d
N
/
d
t
{\displaystyle f=\mathrm {d} N/\mathrm {d} t\,\!}
For non-FM waves this reduces to:
f
=
Δ
N
/
Δ
t
{\displaystyle f=\Delta N/\Delta t\,\!}
In practice N is set to 1 cycle and t = T = time period for 1 cycle, to obtain the more useful relation:
f
=
1
/
T
{\displaystyle f=1/T\,\!}
Hz = s−1
[T]−1
Angular frequency / pulsatance
ω
ω
=
2
π
f
=
2
π
/
T
{\displaystyle \omega =2\pi f=2\pi /T\,\!}
Hz = s−1
[T]−1
Oscillatory velocity
v , vt , v
Longitudinal waves:
v
=
e
^
∥
(
∂
A
/
∂
t
)
{\displaystyle \mathbf {v} =\mathbf {\hat {e}} _{\parallel }\left(\partial A/\partial t\right)\,\!}
Transverse waves:
v
=
e
^
⊥
(
∂
A
/
∂
t
)
{\displaystyle \mathbf {v} =\mathbf {\hat {e}} _{\bot }\left(\partial A/\partial t\right)\,\!}
m s−1
[L][T]−1
Oscillatory acceleration
a , at
Longitudinal waves:
a
=
e
^
∥
(
∂
2
A
/
∂
t
2
)
{\displaystyle \mathbf {a} =\mathbf {\hat {e}} _{\parallel }\left(\partial ^{2}A/\partial t^{2}\right)\,\!}
Transverse waves:
a
=
e
^
⊥
(
∂
2
A
/
∂
t
2
)
{\displaystyle \mathbf {a} =\mathbf {\hat {e}} _{\bot }\left(\partial ^{2}A/\partial t^{2}\right)\,\!}
m s−2
[L][T]−2
Path length difference between two waves
L , ΔL , Δx , Δr
r
=
r
2
−
r
1
{\displaystyle \mathbf {r} =\mathbf {r} _{2}-\mathbf {r} _{1}\,\!}
m
[L]
Phase velocity
vp
General definition:
v
p
=
e
^
∥
(
Δ
r
/
Δ
t
)
{\displaystyle \mathbf {v} _{\mathrm {p} }=\mathbf {\hat {e}} _{\parallel }\left(\Delta r/\Delta t\right)\,\!}
In practice reduces to the useful form:
v
p
=
λ
f
e
^
∥
=
(
ω
/
k
)
e
^
∥
{\displaystyle \mathbf {v} _{\mathrm {p} }=\lambda f\mathbf {\hat {e}} _{\parallel }=\left(\omega /k\right)\mathbf {\hat {e}} _{\parallel }\,\!}
m s−1
[L][T]−1
(Longitudinal) group velocity
vg
v
g
=
e
^
∥
(
∂
ω
/
∂
k
)
{\displaystyle \mathbf {v} _{\mathrm {g} }=\mathbf {\hat {e}} _{\parallel }\left(\partial \omega /\partial k\right)\,\!}
m s−1
[L][T]−1
Time delay, time lag/lead
Δt
Δ
t
=
t
2
−
t
1
{\displaystyle \Delta t=t_{2}-t_{1}\,\!}
s
[T]
Phase difference
δ , Δε , Δϕ
Δ
ϕ
=
ϕ
2
−
ϕ
1
{\displaystyle \Delta \phi =\phi _{2}-\phi _{1}\,\!}
rad
dimensionless
Phase
No standard symbol
k
⋅
r
∓
ω
t
+
ϕ
=
2
π
N
{\displaystyle \mathbf {k} \cdot \mathbf {r} \mp \omega t+\phi =2\pi N\,\!}
upper sign: wave propagation in +r direction
lower sign: wave propagation in −r direction
Phase angle can lag if: ϕ > 0
or lead if: ϕ < 0.
rad
dimensionless
Relation between space, time, angle analogues used to describe the phase:
Δ
r
λ
=
Δ
t
T
=
ϕ
2
π
{\displaystyle {\frac {\Delta r}{\lambda }}={\frac {\Delta t}{T}}={\frac {\phi }{2\pi }}\,\!}
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI units
Dimension
AM index :
h , hAM
h
A
M
=
A
/
A
m
{\displaystyle h_{AM}=A/A_{m}\,\!}
A = carrier amplitude
Am = peak amplitude of a component in the modulating signal
dimensionless
dimensionless
FM index :
hFM
h
F
M
=
Δ
f
/
f
m
{\displaystyle h_{FM}=\Delta f/f_{m}\,\!}
f = max. deviation of the instantaneous frequency from the carrier frequency
f m = peak frequency of a component in the modulating signal
dimensionless
dimensionless
PM index :
hPM
h
P
M
=
Δ
ϕ
{\displaystyle h_{PM}=\Delta \phi \,\!}
ϕ = peak phase deviation
dimensionless
dimensionless
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI units
Dimension
Acoustic impedance
Z
Z
=
ρ
v
{\displaystyle Z=\rho v\,\!}
v = speed of sound, ρ = volume density of medium
kg m−2 s−1
[M] [L]−2 [T]−1
Specific acoustic impedance
z
z
=
Z
S
{\displaystyle z=ZS\,\!}
S = surface area
kg s−1
[M] [T]−1
Sound Level
β
β
=
(
d
B
)
10
log
|
I
I
0
|
{\displaystyle \beta =\left(\mathrm {dB} \right)10\log \left|{\frac {I}{I_{0}}}\right|\,\!}
dimensionless
dimensionless
In what follows n, m are any integers (Z = set of integers );
n
,
m
∈
Z
{\displaystyle n,m\in \mathbf {Z} \,\!}
Physical situation
Nomenclature
Equations
Harmonic frequencies
fn = nth mode of vibration, nth harmonic, (n-1)th overtone
f
n
=
v
λ
n
=
n
v
2
L
=
n
f
1
{\displaystyle f_{n}={\frac {v}{\lambda _{n}}}={\frac {nv}{2L}}=nf_{1}\,\!}
Physical situation
Nomenclature
Equations
Average wave power
P 0 = Sound power due to source
⟨
P
⟩
=
μ
v
ω
2
x
m
2
/
2
{\displaystyle \langle P\rangle =\mu v\omega ^{2}x_{m}^{2}/2\,\!}
Sound intensity
Ω = Solid angle
I
=
P
0
/
(
Ω
r
2
)
{\displaystyle I=P_{0}/(\Omega r^{2})\,\!}
I
=
P
/
A
=
ρ
v
ω
2
s
m
2
/
2
{\displaystyle I=P/A=\rho v\omega ^{2}s_{m}^{2}/2\,\!}
Acoustic beat frequency
f 1 , f 2 = frequencies of two waves (nearly equal amplitudes)
f
b
e
a
t
=
|
f
2
−
f
1
|
{\displaystyle f_{\mathrm {beat} }=\left|f_{2}-f_{1}\right|\,\!}
Doppler effect for mechanical waves
V = speed of sound wave in mediumf0 = Source frequencyfr = Receiver frequencyv0 = Source velocityvr = Receiver velocity
f
r
=
f
0
V
±
v
r
V
∓
v
0
{\displaystyle f_{r}=f_{0}{\frac {V\pm v_{r}}{V\mp v_{0}}}\,\!}
Mach cone angle (Supersonic shockwave, sonic boom)
v = speed of bodyvs = local speed of soundθ = angle between direction of travel and conic envelope of superimposed wavefronts
sin
θ
=
v
v
s
{\displaystyle \sin \theta ={\frac {v}{v_{s}}}\,\!}
Acoustic pressure and displacement amplitudes
p 0 = pressure amplitudes 0 = displacement amplitudev = speed of soundρ = local density of medium
p
0
=
(
v
ρ
ω
)
s
0
{\displaystyle p_{0}=\left(v\rho \omega \right)s_{0}\,\!}
Wave functions for sound
Acoustic beats
s
=
[
2
s
0
cos
(
ω
′
t
)
]
cos
(
ω
t
)
{\displaystyle s=\left[2s_{0}\cos \left(\omega 't\right)\right]\cos \left(\omega t\right)\,\!}
Sound displacement function
s
=
s
0
cos
(
k
r
−
ω
t
)
{\displaystyle s=s_{0}\cos(kr-\omega t)\,\!}
Sound pressure-variation
p
=
p
0
sin
(
k
r
−
ω
t
)
{\displaystyle p=p_{0}\sin(kr-\omega t)\,\!}
Gravitational waves [ edit ] Gravitational radiation for two orbiting bodies in the low-speed limit.[ 11]
Physical situation
Nomenclature
Equations
Radiated power
P = Radiated power from system,t = time,r = separation between centres-of-massm 1 , m 2 = masses of the orbiting bodies
P
=
d
E
d
t
=
−
32
5
G
4
c
5
(
m
1
m
2
)
2
(
m
1
+
m
2
)
r
5
{\displaystyle P={\frac {\mathrm {d} E}{\mathrm {d} t}}=-{\frac {32}{5}}\,{\frac {G^{4}}{c^{5}}}\,{\frac {(m_{1}m_{2})^{2}(m_{1}+m_{2})}{r^{5}}}}
Orbital radius decay
d
r
d
t
=
−
64
5
G
3
c
5
(
m
1
m
2
)
(
m
1
+
m
2
)
r
3
{\displaystyle {\frac {\mathrm {d} r}{\mathrm {d} t}}=-{\frac {64}{5}}{\frac {G^{3}}{c^{5}}}{\frac {(m_{1}m_{2})(m_{1}+m_{2})}{r^{3}}}\ }
Orbital lifetime
r 0 = initial distance between the orbiting bodies
t
=
5
256
c
5
G
3
r
0
4
(
m
1
m
2
)
(
m
1
+
m
2
)
{\displaystyle t={\frac {5}{256}}{\frac {c^{5}}{G^{3}}}{\frac {r_{0}^{4}}{(m_{1}m_{2})(m_{1}+m_{2})}}\ }
[ edit ] A common misconception occurs between phase velocity and group velocity (analogous to centres of mass and gravity). They happen to be equal in non-dispersive media. In dispersive media the phase velocity is not necessarily the same as the group velocity. The phase velocity varies with frequency.
The phase velocity is the rate at which the phase of the wave propagates in space.
The group velocity is the rate at which the wave envelope, i.e. the changes in amplitude, propagates. The wave envelope is the profile of the wave amplitudes; all transverse displacements are bound by the envelope profile. Intuitively the wave envelope is the "global profile" of the wave, which "contains" changing "local profiles inside the global profile". Each propagates at generally different speeds determined by the important function called the dispersion relation ω (k ) is standard, since the phase velocity ω /k and the group velocity dω /dk usually have convenient representations by this function.
General wave functions [ edit ]
Physical situation
Nomenclature
Wave equation
General solution/s
Non-dispersive Wave Equation in 3d
A = amplitude as function of position and time
∇
2
A
=
1
v
∥
2
∂
2
A
∂
t
2
{\displaystyle \nabla ^{2}A={\frac {1}{v_{\parallel }^{2}}}{\frac {\partial ^{2}A}{\partial t^{2}}}\,\!}
A
(
r
,
t
)
=
A
(
x
−
v
∥
t
)
{\displaystyle A\left(\mathbf {r} ,t\right)=A\left(x-v_{\parallel }t\right)\,\!}
Exponentially damped waveform
A 0 = Initial amplitude at time t = 0b = damping parameter
A
=
A
0
e
−
b
t
sin
(
k
x
−
ω
t
+
ϕ
)
{\displaystyle A=A_{0}e^{-bt}\sin \left(kx-\omega t+\phi \right)\,\!}
Korteweg–de Vries equation [ 12] α = constant
∂
y
∂
t
+
α
y
∂
y
∂
x
+
∂
3
y
∂
x
3
=
0
{\displaystyle {\frac {\partial y}{\partial t}}+\alpha y{\frac {\partial y}{\partial x}}+{\frac {\partial ^{3}y}{\partial x^{3}}}=0\,\!}
A
(
x
,
t
)
=
3
v
∥
α
s
e
c
h
2
[
v
∥
2
(
x
−
v
∥
t
)
]
{\displaystyle A(x,t)={\frac {3v_{\parallel }}{\alpha }}\mathrm {sech} ^{2}\left[{\frac {\sqrt {v_{\parallel }}}{2}}\left(x-v_{\parallel }t\right)\right]\,\!}
Sinusoidal solutions to the 3d wave equation [ edit ] N different sinusoidal waves Complex amplitude of wave n
A
n
=
|
A
n
|
e
i
(
k
n
⋅
r
−
ω
n
t
+
ϕ
n
)
{\displaystyle A_{n}=\left|A_{n}\right|e^{i\left(\mathbf {k} _{\mathrm {n} }\cdot \mathbf {r} -\omega _{n}t+\phi _{n}\right)}\,\!}
Resultant complex amplitude of all N waves
A
=
∑
n
=
1
N
A
n
{\displaystyle A=\sum _{n=1}^{N}A_{n}\,\!}
Modulus of amplitude
A
=
A
A
∗
=
∑
n
=
1
N
∑
m
=
1
N
|
A
n
|
|
A
m
|
cos
[
(
k
n
−
k
m
)
⋅
r
+
(
ω
n
−
ω
m
)
t
+
(
ϕ
n
−
ϕ
m
)
]
{\displaystyle A={\sqrt {AA^{*}}}={\sqrt {\sum _{n=1}^{N}\sum _{m=1}^{N}\left|A_{n}\right|\left|A_{m}\right|\cos \left[\left(\mathbf {k} _{n}-\mathbf {k} _{m}\right)\cdot \mathbf {r} +\left(\omega _{n}-\omega _{m}\right)t+\left(\phi _{n}-\phi _{m}\right)\right]}}\,\!}
The transverse displacements are simply the real parts of the complex amplitudes.
1-dimensional corollaries for two sinusoidal waves
The following may be deduced by applying the principle of superposition to two sinusoidal waves, using trigonometric identities. The angle addition and sum-to-product trigonometric formulae are useful; in more advanced work complex numbers and fourier series and transforms are used.
Wavefunction
Nomenclature
Superposition
Resultant
Standing wave
y
1
+
y
2
=
A
sin
(
k
x
−
ω
t
)
+
A
sin
(
k
x
+
ω
t
)
{\displaystyle {\begin{aligned}y_{1}+y_{2}&=A\sin \left(kx-\omega t\right)\\&+A\sin \left(kx+\omega t\right)\end{aligned}}\,\!}
y
=
2
A
sin
(
k
x
)
cos
(
ω
t
)
{\displaystyle y=2A\sin \left(kx\right)\cos \left(\omega t\right)\,\!}
Beats
⟨
ω
⟩
=
ω
1
+
ω
2
2
{\displaystyle \langle \omega \rangle ={\frac {\omega _{1}+\omega _{2}}{2}}\,\!}
⟨
k
⟩
=
k
1
+
k
2
2
{\displaystyle \langle k\rangle ={\frac {k_{1}+k_{2}}{2}}\,\!}
Δ
ω
=
ω
1
−
ω
2
{\displaystyle \Delta \omega =\omega _{1}-\omega _{2}\,\!}
Δ
k
=
k
1
−
k
2
{\displaystyle \Delta k=k_{1}-k_{2}\,\!}
y
1
+
y
2
=
A
sin
(
k
1
x
−
ω
1
t
)
+
A
sin
(
k
2
x
+
ω
2
t
)
{\displaystyle {\begin{aligned}y_{1}+y_{2}&=A\sin \left(k_{1}x-\omega _{1}t\right)\\&+A\sin \left(k_{2}x+\omega _{2}t\right)\end{aligned}}\,\!}
y
=
2
A
sin
(
⟨
k
⟩
x
−
⟨
ω
⟩
t
)
cos
(
Δ
k
2
x
−
Δ
ω
2
t
)
{\displaystyle y=2A\sin \left(\langle k\rangle x-\langle \omega \rangle t\right)\cos \left({\frac {\Delta k}{2}}x-{\frac {\Delta \omega }{2}}t\right)\,\!}
Coherent interference
y
1
+
y
2
=
2
A
sin
(
k
x
−
ω
t
)
+
A
sin
(
k
x
+
ω
t
+
ϕ
)
{\displaystyle {\begin{aligned}y_{1}+y_{2}&=2A\sin \left(kx-\omega t\right)\\&+A\sin \left(kx+\omega t+\phi \right)\end{aligned}}\,\!}
y
=
2
A
cos
(
ϕ
2
)
sin
(
k
x
−
ω
t
+
ϕ
2
)
{\displaystyle y=2A\cos \left({\frac {\phi }{2}}\right)\sin \left(kx-\omega t+{\frac {\phi }{2}}\right)\,\!}
Lorentz force on a charged particle (of charge q ) in motion (velocity v ), used as the definition of the E fieldB field Here subscripts e and m are used to differ between electric and magnetic charges . The definitions for monopoles are of theoretical interest, although real magnetic dipoles can be described using pole strengths. There are two possible units for monopole strength, Wb (Weber) and A m (Ampere metre). Dimensional analysis shows that magnetic charges relate by qm (Wb) = μ 0 qm (Am).
Electric quantities [ edit ] Continuous charge distribution. The volume charge density ρ is the amount of charge per unit volume (cube), surface charge density σ is amount per unit surface area (circle) with outward unit normal n̂ , d is the dipole moment between two point charges, the volume density of these is the polarization density P . Position vector r is a point to calculate the electric field; r′ is a point in the charged object. Contrary to the strong analogy between (classical) gravitation and electrostatics , there are no "centre of charge" or "centre of electrostatic attraction" analogues.
Electric transport
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI units
Dimension
Linear, surface, volumetric charge density
λe for Linear, σe for surface, ρe for volume.
q
e
=
∫
λ
e
d
ℓ
{\displaystyle q_{e}=\int \lambda _{e}\mathrm {d} \ell }
q
e
=
∬
σ
e
d
S
{\displaystyle q_{e}=\iint \sigma _{e}\mathrm {d} S}
q
e
=
∭
ρ
e
d
V
{\displaystyle q_{e}=\iiint \rho _{e}\mathrm {d} V}
C m−n , n = 1, 2, 3
[I][T][L]−n
Capacitance
C
C
=
d
q
/
d
V
{\displaystyle C=\mathrm {d} q/\mathrm {d} V\,\!}
V = voltage, not volume.
F = C V−1
[I]2 [T]4 [L]−2 [M]−1
Electric current
I
I
=
d
q
/
d
t
{\displaystyle I=\mathrm {d} q/\mathrm {d} t\,\!}
A
[I]
Electric current density
J
I
=
J
⋅
d
S
{\displaystyle I=\mathbf {J} \cdot \mathrm {d} \mathbf {S} }
A m−2
[I][L]−2
Displacement current density
J d
J
d
=
ϵ
0
(
∂
E
/
∂
t
)
=
∂
D
/
∂
t
{\displaystyle \mathbf {J} _{\mathrm {d} }=\epsilon _{0}\left(\partial \mathbf {E} /\partial t\right)=\partial \mathbf {D} /\partial t\,\!}
A m−2
[I][L]−2
Convection current density
J c
J
c
=
ρ
v
{\displaystyle \mathbf {J} _{\mathrm {c} }=\rho \mathbf {v} \,\!}
A m−2
[I][L]−2
Electric fields
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI units
Dimension
Electric field , field strength, flux density, potential gradient
E
E
=
F
/
q
{\displaystyle \mathbf {E} =\mathbf {F} /q\,\!}
N C−1 = V m−1
[M][L][T]−3 [I]−1
Electric flux
ΦE
Φ
E
=
∫
S
E
⋅
d
A
{\displaystyle \Phi _{E}=\int _{S}\mathbf {E} \cdot \mathrm {d} \mathbf {A} \,\!}
N m2 C−1
[M][L]3 [T]−3 [I]−1
Absolute permittivity ;
ε
ϵ
=
ϵ
r
ϵ
0
{\displaystyle \epsilon =\epsilon _{r}\epsilon _{0}\,\!}
F m−1
[I]2 [T]4 [M]−1 [L]−3
Electric dipole moment
p
p
=
q
a
{\displaystyle \mathbf {p} =q\mathbf {a} \,\!}
a = charge separation directed from -ve to +ve charge
C m
[I][T][L]
Electric Polarization, polarization density
P
P
=
d
⟨
p
⟩
/
d
V
{\displaystyle \mathbf {P} =\mathrm {d} \langle \mathbf {p} \rangle /\mathrm {d} V\,\!}
C m−2
[I][T][L]−2
Electric displacement field , flux density
D
D
=
ϵ
E
=
ϵ
0
E
+
P
{\displaystyle \mathbf {D} =\epsilon \mathbf {E} =\epsilon _{0}\mathbf {E} +\mathbf {P} \,}
C m−2
[I][T][L]−2
Electric displacement flux
ΦD
Φ
D
=
∫
S
D
⋅
d
A
{\displaystyle \Phi _{D}=\int _{S}\mathbf {D} \cdot \mathrm {d} \mathbf {A} \,\!}
C
[I][T]
Absolute electric potential , EM scalar potential relative to point
r
0
{\displaystyle r_{0}\,\!}
r
0
=
∞
{\displaystyle r_{0}=\infty \,\!}
Practical:
r
0
=
R
e
a
r
t
h
{\displaystyle r_{0}=R_{\mathrm {earth} }\,\!}
φ ,V
V
=
−
W
∞
r
q
=
−
1
q
∫
∞
r
F
⋅
d
r
=
−
∫
r
1
r
2
E
⋅
d
r
{\displaystyle V=-{\frac {W_{\infty r}}{q}}=-{\frac {1}{q}}\int _{\infty }^{r}\mathbf {F} \cdot \mathrm {d} \mathbf {r} =-\int _{r_{1}}^{r_{2}}\mathbf {E} \cdot \mathrm {d} \mathbf {r} \,\!}
V = J C−1
[M] [L]2 [T]−3 [I]−1
Voltage , Electric potential difference
Δφ ,ΔV
Δ
V
=
−
Δ
W
q
=
−
1
q
∫
r
1
r
2
F
⋅
d
r
=
−
∫
r
1
r
2
E
⋅
d
r
{\displaystyle \Delta V=-{\frac {\Delta W}{q}}=-{\frac {1}{q}}\int _{r_{1}}^{r_{2}}\mathbf {F} \cdot \mathrm {d} \mathbf {r} =-\int _{r_{1}}^{r_{2}}\mathbf {E} \cdot \mathrm {d} \mathbf {r} \,\!}
V = J C−1
[M] [L]2 [T]−3 [I]−1
Magnetic quantities [ edit ] Magnetic transport
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI units
Dimension
Linear, surface, volumetric pole density
λm for Linear, σm for surface, ρm for volume.
q
m
=
∫
λ
m
d
ℓ
{\displaystyle q_{m}=\int \lambda _{m}\mathrm {d} \ell }
q
m
=
∬
σ
m
d
S
{\displaystyle q_{m}=\iint \sigma _{m}\mathrm {d} S}
q
m
=
∭
ρ
m
d
V
{\displaystyle q_{m}=\iiint \rho _{m}\mathrm {d} V}
Wb m−n
(−n + 1) ,
n = 1, 2, 3
[L]2 [M][T]−2 [I]−1 (Wb)
Monopole current
Im
I
m
=
d
q
m
/
d
t
{\displaystyle I_{m}=\mathrm {d} q_{m}/\mathrm {d} t\,\!}
Wb s−1
−1
[L]2 [M][T]−3 [I]−1 (Wb)
−1 (Am)
Monopole current density
J m
I
=
∬
J
m
⋅
d
A
{\displaystyle I=\iint \mathbf {J} _{\mathrm {m} }\cdot \mathrm {d} \mathbf {A} }
Wb s−1 m−2
−1 s−1
[M][T]−3 [I]−1 (Wb)
−1 [T]−1 (Am)
Magnetic fields
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI units
Dimension
Magnetic field , field strength, flux density, induction field
B
F
=
q
e
(
v
×
B
)
{\displaystyle \mathbf {F} =q_{e}\left(\mathbf {v} \times \mathbf {B} \right)\,\!}
T = N A−1 m−1 = Wb m−2
[M][T]−2 [I]−1
Magnetic potential , EM vector potential
A
B
=
∇
×
A
{\displaystyle \mathbf {B} =\nabla \times \mathbf {A} }
T m = N A−1 = Wb m3
[M][L][T]−2 [I]−1
Magnetic flux
ΦB
Φ
B
=
∫
S
B
⋅
d
A
{\displaystyle \Phi _{B}=\int _{S}\mathbf {B} \cdot \mathrm {d} \mathbf {A} \,\!}
Wb = T m2
[L]2 [M][T]−2 [I]−1
Magnetic permeability
μ
{\displaystyle \mu \,\!}
μ
=
μ
r
μ
0
{\displaystyle \mu \ =\mu _{r}\,\mu _{0}\,\!}
V·s·A−1 ·m−1 = N·A−2 = T·m·A−1 = Wb·A−1 ·m−1
[M][L][T]−2 [I]−2
Magnetic moment , magnetic dipole moment
m , μB , Π
Two definitions are possible:
using pole strengths,
m
=
q
m
a
{\displaystyle \mathbf {m} =q_{m}\mathbf {a} \,\!}
using currents:
m
=
N
I
A
n
^
{\displaystyle \mathbf {m} =NIA\mathbf {\hat {n}} \,\!}
a = pole separation
N is the number of turns of conductor
A m2
[I][L]2
Magnetization
M
M
=
d
⟨
m
⟩
/
d
V
{\displaystyle \mathbf {M} =\mathrm {d} \langle \mathbf {m} \rangle /\mathrm {d} V\,\!}
A m−1
[I] [L]−1
Magnetic field intensity, (AKA field strength)
H
Two definitions are possible:
most common:
B
=
μ
H
=
μ
0
(
H
+
M
)
{\displaystyle \mathbf {B} =\mu \mathbf {H} =\mu _{0}\left(\mathbf {H} +\mathbf {M} \right)\,}
using pole strengths,[ 13]
H
=
F
/
q
m
{\displaystyle \mathbf {H} =\mathbf {F} /q_{m}\,}
A m−1
[I] [L]−1
Intensity of magnetization , magnetic polarization
I , J
I
=
μ
0
M
{\displaystyle \mathbf {I} =\mu _{0}\mathbf {M} \,\!}
T = N A−1 m−1 = Wb m−2
[M][T]−2 [I]−1
Self Inductance
L
Two equivalent definitions are possible:
L
=
N
(
d
Φ
/
d
I
)
{\displaystyle L=N\left(\mathrm {d} \Phi /\mathrm {d} I\right)\,\!}
L
(
d
I
/
d
t
)
=
−
N
V
{\displaystyle L\left(\mathrm {d} I/\mathrm {d} t\right)=-NV\,\!}
H = Wb A−1
[L]2 [M] [T]−2 [I]−2
Mutual inductance
M
Again two equivalent definitions are possible:
M
1
=
N
(
d
Φ
2
/
d
I
1
)
{\displaystyle M_{1}=N\left(\mathrm {d} \Phi _{2}/\mathrm {d} I_{1}\right)\,\!}
M
(
d
I
2
/
d
t
)
=
−
N
V
1
{\displaystyle M\left(\mathrm {d} I_{2}/\mathrm {d} t\right)=-NV_{1}\,\!}
1,2 subscripts refer to two conductors/inductors mutually inducing voltage/ linking magnetic flux through each other. They can be interchanged for the required conductor/inductor;
M
2
=
N
(
d
Φ
1
/
d
I
2
)
{\displaystyle M_{2}=N\left(\mathrm {d} \Phi _{1}/\mathrm {d} I_{2}\right)\,\!}
M
(
d
I
1
/
d
t
)
=
−
N
V
2
{\displaystyle M\left(\mathrm {d} I_{1}/\mathrm {d} t\right)=-NV_{2}\,\!}
H = Wb A−1
[L]2 [M] [T]−2 [I]−2
Gyromagnetic ratio (for charged particles in a magnetic field)
γ
ω
=
γ
B
{\displaystyle \omega =\gamma B\,\!}
Hz T−1
[M]−1 [T][I]
DC circuits, general definitions
AC circuits
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI units
Dimension
Resistive load voltage
VR
V
R
=
I
R
R
{\displaystyle V_{R}=I_{R}R\,\!}
V = J C−1
[M] [L]2 [T]−3 [I]−1
Capacitive load voltage
VC
V
C
=
I
C
X
C
{\displaystyle V_{C}=I_{C}X_{C}\,\!}
V = J C−1
[M] [L]2 [T]−3 [I]−1
Inductive load voltage
VL
V
L
=
I
L
X
L
{\displaystyle V_{L}=I_{L}X_{L}\,\!}
V = J C−1
[M] [L]2 [T]−3 [I]−1
Capacitive reactance
XC
X
C
=
1
ω
d
C
{\displaystyle X_{C}={\frac {1}{\omega _{\mathrm {d} }C}}\,\!}
Ω−1 m−1
[I]2 [T]3 [M]−2 [L]−2
Inductive reactance
XL
X
L
=
ω
d
L
{\displaystyle X_{L}=\omega _{d}L\,\!}
Ω−1 m−1
[I]2 [T]3 [M]−2 [L]−2
AC electrical impedance
Z
V
=
I
Z
{\displaystyle V=IZ\,\!}
Z
=
R
2
+
(
X
L
−
X
C
)
2
{\displaystyle Z={\sqrt {R^{2}+\left(X_{L}-X_{C}\right)^{2}}}\,\!}
Ω−1 m−1
[I]2 [T]3 [M]−2 [L]−2
Phase constant
δ, φ
tan
ϕ
=
X
L
−
X
C
R
{\displaystyle \tan \phi ={\frac {X_{L}-X_{C}}{R}}\,\!}
dimensionless
dimensionless
AC peak current
I 0
I
0
=
I
r
m
s
2
{\displaystyle I_{0}=I_{\mathrm {rms} }{\sqrt {2}}\,\!}
A
[I]
AC root mean square current
I rms
I
r
m
s
=
1
T
∫
0
T
[
I
(
t
)
]
2
d
t
{\displaystyle I_{\mathrm {rms} }={\sqrt {{\frac {1}{T}}\int _{0}^{T}\left[I\left(t\right)\right]^{2}\mathrm {d} t}}\,\!}
A
[I]
AC peak voltage
V 0
V
0
=
V
r
m
s
2
{\displaystyle V_{0}=V_{\mathrm {rms} }{\sqrt {2}}\,\!}
V = J C−1
[M] [L]2 [T]−3 [I]−1
AC root mean square voltage
V rms
V
r
m
s
=
1
T
∫
0
T
[
V
(
t
)
]
2
d
t
{\displaystyle V_{\mathrm {rms} }={\sqrt {{\frac {1}{T}}\int _{0}^{T}\left[V\left(t\right)\right]^{2}\mathrm {d} t}}\,\!}
V = J C−1
[M] [L]2 [T]−3 [I]−1
AC emf, root mean square
E
r
m
s
,
⟨
E
⟩
{\displaystyle {\mathcal {E}}_{\mathrm {rms} },{\sqrt {\langle {\mathcal {E}}\rangle }}\,\!}
E
r
m
s
=
E
m
/
2
{\displaystyle {\mathcal {E}}_{\mathrm {rms} }={\mathcal {E}}_{\mathrm {m} }/{\sqrt {2}}\,\!}
V = J C−1
[M] [L]2 [T]−3 [I]−1
AC average power
⟨
P
⟩
{\displaystyle \langle P\rangle \,\!}
⟨
P
⟩
=
E
I
r
m
s
cos
ϕ
{\displaystyle \langle P\rangle ={\mathcal {E}}I_{\mathrm {rms} }\cos \phi \,\!}
W = J s−1
[M] [L]2 [T]−3
Capacitive time constant
τC
τ
C
=
R
C
{\displaystyle \tau _{C}=RC\,\!}
s
[T]
Inductive time constant
τL
τ
L
=
L
/
R
{\displaystyle \tau _{L}=L/R\,\!}
s
[T]
Quantity (common name/s)
(Common) symbol/s
Defining equation
SI units
Dimension
Magnetomotive force , mmf
F ,
F
,
M
{\displaystyle {\mathcal {F}},{\mathcal {M}}}
M
=
N
I
{\displaystyle {\mathcal {M}}=NI}
N = number of turns of conductor
A
[I]
General Classical Equations
Physical situation
Equations
Electric potential gradient and field
E
=
−
∇
V
{\displaystyle \mathbf {E} =-\nabla V}
Δ
V
=
−
∫
r
1
r
2
E
⋅
d
r
{\displaystyle \Delta V=-\int _{r_{1}}^{r_{2}}\mathbf {E} \cdot d\mathbf {r} \,\!}
Point charge
E
=
q
4
π
ϵ
0
|
r
|
2
r
^
{\displaystyle \mathbf {E} ={\frac {q}{4\pi \epsilon _{0}\left|\mathbf {r} \right|^{2}}}\mathbf {\hat {r}} \,\!}
At a point in a local array of point charges
E
=
∑
E
i
=
1
4
π
ϵ
0
∑
i
q
i
|
r
i
−
r
|
2
r
^
i
{\displaystyle \mathbf {E} =\sum \mathbf {E} _{i}={\frac {1}{4\pi \epsilon _{0}}}\sum _{i}{\frac {q_{i}}{\left|\mathbf {r} _{i}-\mathbf {r} \right|^{2}}}\mathbf {\hat {r}} _{i}\,\!}
At a point due to a continuum of charge
E
=
1
4
π
ϵ
0
∫
V
r
ρ
d
V
|
r
|
3
{\displaystyle \mathbf {E} ={\frac {1}{4\pi \epsilon _{0}}}\int _{V}{\frac {\mathbf {r} \rho \mathrm {d} V}{\left|\mathbf {r} \right|^{3}}}\,\!}
Electrostatic torque and potential energy due to non-uniform fields and dipole moments
τ
=
∫
V
d
p
×
E
{\displaystyle {\boldsymbol {\tau }}=\int _{V}\mathrm {d} \mathbf {p} \times \mathbf {E} }
U
=
−
∫
V
d
p
⋅
E
{\displaystyle U=-\int _{V}\mathrm {d} \mathbf {p} \cdot \mathbf {E} }
Magnetic fields and moments [ edit ] General classical equations
Physical situation
Equations
Magnetic potential, EM vector potential
B
=
∇
×
A
{\displaystyle \mathbf {B} =\nabla \times \mathbf {A} }
Due to a magnetic moment
A
=
μ
0
4
π
m
×
r
|
r
|
3
{\displaystyle \mathbf {A} ={\frac {\mu _{0}}{4\pi }}{\frac {\mathbf {m} \times \mathbf {r} }{\left|\mathbf {r} \right|^{3}}}}
B
(
r
)
=
∇
×
A
=
μ
0
4
π
(
3
r
(
m
⋅
r
)
|
r
|
5
−
m
|
r
|
3
)
{\displaystyle \mathbf {B} ({\mathbf {r} })=\nabla \times {\mathbf {A} }={\frac {\mu _{0}}{4\pi }}\left({\frac {3\mathbf {r} (\mathbf {m} \cdot \mathbf {r} )}{\left|\mathbf {r} \right|^{5}}}-{\frac {\mathbf {m} }{\left|\mathbf {r} \right|^{3}}}\right)}
Magnetic moment due to a current distribution
m
=
1
2
∫
V
r
×
J
d
V
{\displaystyle \mathbf {m} ={\frac {1}{2}}\int _{V}\mathbf {r} \times \mathbf {J} \mathrm {d} V}
Magnetostatic torque and potential energy due to non-uniform fields and dipole moments
τ
=
∫
V
d
m
×
B
{\displaystyle {\boldsymbol {\tau }}=\int _{V}\mathrm {d} \mathbf {m} \times \mathbf {B} }
U
=
−
∫
V
d
m
⋅
B
{\displaystyle U=-\int _{V}\mathrm {d} \mathbf {m} \cdot \mathbf {B} }
Electric circuits and electronics [ edit ] Below N = number of conductors or circuit components. Subscript net refers to the equivalent and resultant property value.
Physical situation
Nomenclature
Series
Parallel
Resistors and conductors
Ri = resistance of resistor or conductor i Gi = conductance of resistor or conductor i
R
n
e
t
=
∑
i
=
1
N
R
i
{\displaystyle R_{\mathrm {net} }=\sum _{i=1}^{N}R_{i}\,\!}
1
G
n
e
t
=
∑
i
=
1
N
1
G
i
{\displaystyle {1 \over G_{\mathrm {net} }}=\sum _{i=1}^{N}{1 \over G_{i}}\,\!}
1
R
n
e
t
=
∑
i
=
1
N
1
R
i
{\displaystyle {1 \over R_{\mathrm {net} }}=\sum _{i=1}^{N}{1 \over R_{i}}\,\!}
G
n
e
t
=
∑
i
=
1
N
G
i
{\displaystyle G_{\mathrm {net} }=\sum _{i=1}^{N}G_{i}\,\!}
Charge, capacitors, currents
Ci = capacitance of capacitor i qi = charge of charge carrier i
q
n
e
t
=
∑
i
=
1
N
q
i
{\displaystyle q_{\mathrm {net} }=\sum _{i=1}^{N}q_{i}\,\!}
1
C
n
e
t
=
∑
i
=
1
N
1
C
i
{\displaystyle {1 \over C_{\mathrm {net} }}=\sum _{i=1}^{N}{1 \over C_{i}}\,\!}
I
n
e
t
=
I
i
{\displaystyle I_{\mathrm {net} }=I_{i}\,\!}
q
n
e
t
=
∑
i
=
1
N
q
i
{\displaystyle q_{\mathrm {net} }=\sum _{i=1}^{N}q_{i}\,\!}
C
n
e
t
=
∑
i
=
1
N
C
i
{\displaystyle C_{\mathrm {net} }=\sum _{i=1}^{N}C_{i}\,\!}
I
n
e
t
=
∑
i
=
1
N
I
i
{\displaystyle I_{\mathrm {net} }=\sum _{i=1}^{N}I_{i}\,\!}
Inductors
Li = self-inductance of inductor i Lij = self-inductance element ij of L matrixMij = mutual inductance between inductors i and j
L
n
e
t
=
∑
i
=
1
N
L
i
{\displaystyle L_{\mathrm {net} }=\sum _{i=1}^{N}L_{i}\,\!}
1
L
n
e
t
=
∑
i
=
1
N
1
L
i
{\displaystyle {1 \over L_{\mathrm {net} }}=\sum _{i=1}^{N}{1 \over L_{i}}\,\!}
V
i
=
∑
j
=
1
N
L
i
j
d
I
j
d
t
{\displaystyle V_{i}=\sum _{j=1}^{N}L_{ij}{\frac {\mathrm {d} I_{j}}{\mathrm {d} t}}\,\!}
Circuit
DC Circuit equations
AC Circuit equations
Series circuit equations
RC circuits
Circuit equation
R
d
q
d
t
+
q
C
=
E
{\displaystyle R{\frac {\mathrm {d} q}{\mathrm {d} t}}+{\frac {q}{C}}={\mathcal {E}}\,\!}
Capacitor charge
q
=
C
E
(
1
−
e
−
t
/
R
C
)
{\displaystyle q=C{\mathcal {E}}\left(1-e^{-t/RC}\right)\,\!}
Capacitor discharge
q
=
C
E
e
−
t
/
R
C
{\displaystyle q=C{\mathcal {E}}e^{-t/RC}\,\!}
RL circuits
Circuit equation
L
d
I
d
t
+
R
I
=
E
{\displaystyle L{\frac {\mathrm {d} I}{\mathrm {d} t}}+RI={\mathcal {E}}\,\!}
Inductor current rise
I
=
E
R
(
1
−
e
−
R
t
/
L
)
{\displaystyle I={\frac {\mathcal {E}}{R}}\left(1-e^{-Rt/L}\right)\,\!}
Inductor current fall
I
=
E
R
e
−
t
/
τ
L
=
I
0
e
−
R
t
/
L
{\displaystyle I={\frac {\mathcal {E}}{R}}e^{-t/\tau _{L}}=I_{0}e^{-Rt/L}\,\!}
LC circuits
Circuit equation
L
d
2
q
d
t
2
+
q
/
C
=
E
{\displaystyle L{\frac {\mathrm {d} ^{2}q}{\mathrm {d} t^{2}}}+q/C={\mathcal {E}}\,\!}
Circuit equation
L
d
2
q
d
t
2
+
q
/
C
=
E
sin
(
ω
0
t
+
ϕ
)
{\displaystyle L{\frac {\mathrm {d} ^{2}q}{\mathrm {d} t^{2}}}+q/C={\mathcal {E}}\sin \left(\omega _{0}t+\phi \right)\,\!}
Circuit resonant frequency
ω
r
e
s
=
1
/
L
C
{\displaystyle \omega _{\mathrm {res} }=1/{\sqrt {LC}}\,\!}
Circuit charge
q
=
q
0
cos
(
ω
t
+
ϕ
)
{\displaystyle q=q_{0}\cos(\omega t+\phi )\,\!}
Circuit current
I
=
−
ω
q
0
sin
(
ω
t
+
ϕ
)
{\displaystyle I=-\omega q_{0}\sin(\omega t+\phi )\,\!}
Circuit electrical potential energy
U
E
=
q
2
/
2
C
=
Q
2
cos
2
(
ω
t
+
ϕ
)
/
2
C
{\displaystyle U_{E}=q^{2}/2C=Q^{2}\cos ^{2}(\omega t+\phi )/2C\,\!}
Circuit magnetic potential energy
U
B
=
Q
2
sin
2
(
ω
t
+
ϕ
)
/
2
C
{\displaystyle U_{B}=Q^{2}\sin ^{2}(\omega t+\phi )/2C\,\!}
RLC Circuits
Circuit equation
L
d
2
q
d
t
2
+
R
d
q
d
t
+
q
C
=
E
{\displaystyle L{\frac {\mathrm {d} ^{2}q}{\mathrm {d} t^{2}}}+R{\frac {\mathrm {d} q}{\mathrm {d} t}}+{\frac {q}{C}}={\mathcal {E}}\,\!}
Circuit equation
L
d
2
q
d
t
2
+
R
d
q
d
t
+
q
C
=
E
sin
(
ω
0
t
+
ϕ
)
{\displaystyle L{\frac {\mathrm {d} ^{2}q}{\mathrm {d} t^{2}}}+R{\frac {\mathrm {d} q}{\mathrm {d} t}}+{\frac {q}{C}}={\mathcal {E}}\sin \left(\omega _{0}t+\phi \right)\,\!}
Circuit charge
q
=
q
0
e
T
−
R
t
/
2
L
cos
(
ω
′
t
+
ϕ
)
{\displaystyle q=q_{0}eT^{-Rt/2L}\cos(\omega 't+\phi )\,\!}
^ Feynman, R.P.; Leighton, R.B.; Sand, M. (1964). "Operators". The Feynman Lectures on Physics Addison-Wesley . pp. 20–7. ISBN 0-201-02115-3 ^ Shankar, R. (1994). Principles of Quantum Mechanics Kluwer Academic /Plenum Publishers . p. 141 . ISBN 978-0-306-44790-7 ^ Keenan, Thermodynamics , Wiley, New York, 1947
^ Physical chemistry, P.W. Atkins, Oxford University Press, 1978, ISBN 0 19 855148 7
^ "Section: Moments and center of mass " .^ R.P. Feynman; R.B. Leighton; M. Sands (1964). Feynman's Lectures on Physics (volume 2) . Addison-Wesley. pp. 31–7. ISBN 978-0-201-02117-2 ^ "Relativity, J.R. Forshaw 2009"
^ "Mechanics, D. Kleppner 2010"
^ "Relativity, J.R. Forshaw 2009"
^ "Relativity, J.R. Forshaw 2009"
^ "Gravitational Radiation" (PDF) . Archived from the original (PDF) on 2012-04-02. Retrieved 2012-09-15 .^ Encyclopaedia of Physics (2nd Edition), R.G. Lerner , G.L. Trigg, VHC publishers, 1991, (Verlagsgesellschaft) 3-527-26954-1, (VHC Inc.) 0-89573-752-3
^ M. Mansfield; C. O'Sullivan (2011). Understanding Physics (2nd ed.). John Wiley & Sons. ISBN 978-0-470-74637-0 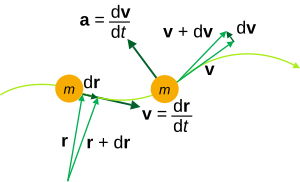















![{\displaystyle {\frac {d}{dt}}{\hat {A}}(t)={\frac {i}{\hbar }}[{\hat {H}},{\hat {A}}(t)]+{\frac {\partial {\hat {A}}(t)}{\partial t}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/53ff70e6aea72bbc04d4ec460a8703495a5f3c42)
![{\displaystyle {\frac {d}{dt}}\langle {\hat {A}}\rangle ={\frac {1}{i\hbar }}\langle [{\hat {A}},{\hat {H}}]\rangle +\left\langle {\frac {\partial {\hat {A}}}{\partial t}}\right\rangle }](https://wikimedia.org/api/rest_v1/media/math/render/svg/c36bf56d57534b1fd67ce51f68de906be35f7e65)





































![{\displaystyle \sigma (A)\sigma (B)\geq {\frac {1}{2}}\langle i[{\hat {A}},{\hat {B}}]\rangle }](https://wikimedia.org/api/rest_v1/media/math/render/svg/e7411e4093e68f746eed92d2171bb2cd536251b8)















































































































































































































































![{\displaystyle {\begin{aligned}\mathbf {Y} &={\frac {d\mathbf {F} }{dt}}={\frac {d^{2}\mathbf {p} }{dt^{2}}}={\frac {d^{2}(m\mathbf {v} )}{dt^{2}}}\\[1ex]&=m\mathbf {j} +\mathbf {2a} {\frac {{\rm {d}}m}{{\rm {d}}t}}+\mathbf {v} {\frac {{\rm {d^{2}}}m}{{\rm {d}}t^{2}}}\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0a298a3dbfef1b35f56011f1b5d941a948e09024)





























































![{\displaystyle \omega ={\sqrt {\frac {g}{L}}}\left[1+\sum _{k=1}^{\infty }{\frac {\prod _{n=1}^{k}\left(2n-1\right)}{\prod _{n=1}^{m}\left(2n\right)}}\sin ^{2n}\Theta \right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2d051a3e661a258eec322d4e38c4560c8013746a)











































![{\displaystyle s=\left[2s_{0}\cos \left(\omega 't\right)\right]\cos \left(\omega t\right)\,\!}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2391651d10c73c1469089652801a9b5cb185944f)


















![{\displaystyle A(x,t)={\frac {3v_{\parallel }}{\alpha }}\mathrm {sech} ^{2}\left[{\frac {\sqrt {v_{\parallel }}}{2}}\left(x-v_{\parallel }t\right)\right]\,\!}](https://wikimedia.org/api/rest_v1/media/math/render/svg/278650bbaf7a9fbd65757104dc4156a44d3106bc)


![{\displaystyle A={\sqrt {AA^{*}}}={\sqrt {\sum _{n=1}^{N}\sum _{m=1}^{N}\left|A_{n}\right|\left|A_{m}\right|\cos \left[\left(\mathbf {k} _{n}-\mathbf {k} _{m}\right)\cdot \mathbf {r} +\left(\omega _{n}-\omega _{m}\right)t+\left(\phi _{n}-\phi _{m}\right)\right]}}\,\!}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a057792fcbc971d9e70e981e77b62c37d5fdee33)

































































![{\displaystyle I_{\mathrm {rms} }={\sqrt {{\frac {1}{T}}\int _{0}^{T}\left[I\left(t\right)\right]^{2}\mathrm {d} t}}\,\!}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a1def0a7f6e77cb4c21b955601a657047a645a97)

![{\displaystyle V_{\mathrm {rms} }={\sqrt {{\frac {1}{T}}\int _{0}^{T}\left[V\left(t\right)\right]^{2}\mathrm {d} t}}\,\!}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2a47cb2bc8518d427829d5d187367748f99a3dcf)















































