Calcium metabolism: Difference between revisions
AntyJusteen (talk | contribs) No edit summary |
|||
| Line 1: | Line 1: | ||
[[Image:Ca-TableImage.png|thumb|350px|[[Calcium]]]] |
[[Image:Ca-TableImage.png|thumb|350px|[[Calcium]]]] |
||
'''Calcium metabolism''' or '''calcium homeostasis''' is the mechanism by which the body maintains adequate [[calcium]] levels. Derangements of this mechanism lead to [[hypercalcemia]] or [[hypocalcemia]], both of which can have important consequences for health. |
'''Calcium metabolism''' or '''calcium homeostasis''' is the mechanism by which the body maintains adequate [[calcium]] levels. Derangements of this mechanism lead to [[hypercalcemia]] or [[hypocalcemia]], both of which can have important consequences for health. When blood calcium level rises above a set point, the thyroid gland releases calcitonin, causing the blood calcium level to fall. When blood calcium level falls below a set point, the parathyroid gland releases parathyroid hormones (PTH), causing the blood calcium level to rise. |
||
==Calcium location and quantity== |
==Calcium location and quantity== |
||
| Line 39: | Line 39: | ||
The [[kidney]] excretes 250 mmol a day in pro-urine, and resorbs 245 mmol, leading to a net loss in the urine of 5 mmol/d. In addition to this, the kidney processes Vitamin D into [[calcitriol]], the active form that is most effective in assisting intestinal absorption. Both processes are stimulated by [[parathyroid hormone]]. |
The [[kidney]] excretes 250 mmol a day in pro-urine, and resorbs 245 mmol, leading to a net loss in the urine of 5 mmol/d. In addition to this, the kidney processes Vitamin D into [[calcitriol]], the active form that is most effective in assisting intestinal absorption. Both processes are stimulated by [[parathyroid hormone]]. |
||
=== |
=== Bones role=== |
||
Although calcium flow to and from the [[bone]] is neutral, about 5 mmol is turned over a day. Bone serves as an important storage point for calcium, as it contains 99% of the total body calcium. Calcium release from bone is regulated by [[parathyroid hormone]]. [[Calcitonin]] stimulates incorporation of calcium in bone, although this process is largely independent of calcitonin. |
Although calcium flow to and from the [[bone]] is neutral, about 5 mmol is turned over a day. Bone serves as an important storage point for calcium, as it contains 99% of the total body calcium. Calcium release from bone is regulated by [[parathyroid hormone]]. [[Calcitonin]] stimulates incorporation of calcium in bone, although this process is largely independent of calcitonin. |
||
Revision as of 04:54, 23 February 2014

Calcium metabolism or calcium homeostasis is the mechanism by which the body maintains adequate calcium levels. Derangements of this mechanism lead to hypercalcemia or hypocalcemia, both of which can have important consequences for health. When blood calcium level rises above a set point, the thyroid gland releases calcitonin, causing the blood calcium level to fall. When blood calcium level falls below a set point, the parathyroid gland releases parathyroid hormones (PTH), causing the blood calcium level to rise.
Calcium location and quantity
Calcium is the most abundant mineral in the human body. The average adult body contains in total approximately 1 kg, 99% in the skeleton in the form of calcium phosphate salts. The extracellular fluid (ECF) contains approximately 22.5 mmol, of which about 9 mmol is in the plasma. Approximately 500 mmol of calcium is exchanged between bone and the ECF over a period of twenty-four hours.[1]
Biological functions
- Structural function: Supporting material in bones. Present as calcium phosphate.
- Signalling function: Intracellular calcium functions as a second messenger for the secretion of some hormones and neurotransmitters. Also acts as an intracellular permeation regulator and mediator of muscle contraction.
- Enzymatic function: Calcium acts as a coenzyme for clotting factors.
Calcium also causes the release of Acetylcholine from pre-synaptic terminals in the transmission of nerve impulses. Calcium causes the contraction of muscles, removing the Triosephosphate isomerase (TPI) subunit from Myosin head which has ATPase activity to cause contraction.
Normal ranges
The plasma level of calcium is closely regulated with a normal total calcium of 2.2-2.6 mmol/L (9-10.5 mg/dL) and a normal ionized calcium of 1.1-1.4 mmol/L (4.5-5.6 mg/dL). The amount of total calcium varies with the level of serum albumin, a protein to which calcium is bound. The biologic effect of calcium is determined by the amount of ionized calcium, rather than the total calcium. Ionized calcium does not vary with the albumin level, and therefore it is useful to measure the ionized calcium level when the serum albumin is not within normal ranges, or when a calcium disorder is suspected despite a normal total calcium level.
Corrected calcium level
One can derive a corrected calcium (also known as adjusted calcium) level, to allow for the change in total calcium due to the change in albumin-bound calcium. This gives an estimate of what the total calcium level would be if the albumin were a specified normal value. Exact formulae used to derive corrected calcium may depend on the analytical methods used for calcium and albumin. However the traditional method of calculating it is shown below.
- Corrected calcium (mg/dL) = measured total Ca (mg/dL) + 0.8 (4.0 - serum albumin [g/dL]), where 4.0 represents the average albumin level in g/dL.
in other words, each 1 g/dL decrease of albumin will decrease 0.8 mg/dL in measured serum Ca and thus 0.8 must be added to the measured Calcium to get a corrected Calcium value.
- Or: Corrected calcium (mmol/L) = measured total Ca (mmol/L) + 0.02 (40 - serum albumin [g/L]), where 40 represents the average albumin level in g/L
in other words, each 1 g/L decrease of albumin, will decrease 0.02 mmol/L in measured serum Ca and thus 0.02 must be added to the measured value to take this into account and get a corrected calcium.
When there is hypoalbuminemia (a lower than normal albumin), the corrected calcium level is higher than the total calcium.
Effector organs
Absorption
About 25 mmol of calcium enters the body in a normal diet. Of this, about 40% (10 mmol) is absorbed in small intestine, and 5 mmol leaves the body in feces, netting 5 mmol of calcium a day.[2]
Calcium is absorbed across the intestinal brush border membrane, passing through ion channels such as TRPV6. Calbindin is a vitamin D-dependent calcium-binding protein inside intestinal epithelial cells which functions together with TRPV6 and calcium pumps (PMCA1) in the basal membrane to actively transport calcium into the body.[3] Active transport of calcium occurs primarily in the duodenum portion of the intestine when calcium intake is low; and through passive paracellular transport occurs in the jejunum and ileum parts when calcium intake is high, independent of Vitamin D level.[4]
Excretion
The kidney excretes 250 mmol a day in pro-urine, and resorbs 245 mmol, leading to a net loss in the urine of 5 mmol/d. In addition to this, the kidney processes Vitamin D into calcitriol, the active form that is most effective in assisting intestinal absorption. Both processes are stimulated by parathyroid hormone.
Bones role
Although calcium flow to and from the bone is neutral, about 5 mmol is turned over a day. Bone serves as an important storage point for calcium, as it contains 99% of the total body calcium. Calcium release from bone is regulated by parathyroid hormone. Calcitonin stimulates incorporation of calcium in bone, although this process is largely independent of calcitonin.
Low calcium intake may also be a risk factor in the development of osteoporosis. In one meta-analysis, the authors found that fifty out of the fifty-two studies that they reviewed showed that calcium intake promoted better bone balance.[5] With a better bone balance, the risk of osteoporosis is lowered.
Interaction with other chemicals
Potential positive interactions
- Vitamin D is an important co-factor in the intestinal absorption of calcium, as it increases the number of calcium binding proteins, involved in calcium absorption through the apical membrane of enterocytes in small intestine. It also promotes re-absorption of calcium in the kidneys.[citation needed]
- Magnesium also plays an important role in calcium absorption by bones. Release of calcium from the sarcoplasmic reticulum is inhibited by magnesium. Thus hypomagnesemia results in an increased intracellular calcium level. This inhibits the release of parathyroid hormone, which can result in hypoparathyroidism and hypocalcemia. Furthermore, it makes skeletal and muscle receptors less sensitive to parathyroid hormone. [citation needed]
- Boron[citation needed]
Potential negative interactions
- "Unesterified long-chain saturated fatty acids, i.e. palmitic acid, have a melting point above body temperature and, with sufficient calcium in the intestinal lumen, form insoluble calcium soaps."[6]
- Sodium binding to calcium[7]
- Phytic acid binding to calcium[8]
- Oxalic acid binding to calcium[9]
- Cortisol[10] binding to calcium[11]
- Low pH food and proteins (the latter promotes gastric acid)[citation needed]
Regulatory organs
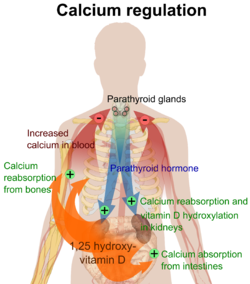
Primarily calcium is regulated by the actions of 1,25-Dihydroxycholecalciferol, parathyroid hormone (PTH) and calcitonin and direct exchange with the bone matrix. Plasma calcium levels are regulated by hormonal and non-hormonal mechanisms. After ingestion of substantial amounts of calcium the short term control that prevents calcium spiking in the serum is absorption by the bone matrix. After about an hour, PTH will be released and not peak for about 8 hours.[13] The PTH is, over time, a very potent regulator of plasma calcium, and controls the conversion of vitamin D into its active form in the kidney. The parathyroid glands are located behind the thyroid, and produce parathyroid hormone in response to low calcium levels.
The parafollicular cells of the thyroid produce calcitonin in response to high calcium levels, but its significance is much smaller than that of PTH.
Pathology
Hypocalcemia and hypercalcemia are both serious medical disorders.
Renal osteodystrophy is a consequence of chronic renal failure related to the calcium metabolism.
Osteoporosis and osteomalacia have been linked to calcium metabolism disorders.
Research into cancer prevention
The role that calcium might have in reducing the rates of colorectal cancer has been the subject of many studies. However, given its modest efficacy, there is no current medical recommendation to use calcium for cancer reduction. Several epidemiological studies suggest that people with high calcium intake have a reduced risk of colorectal cancer. These observations have been confirmed by experimental studies in volunteers and in rodents. One large scale clinical trial shows that 1.2 g calcium each day reduces, modestly, intestinal polyps recurrence in volunteers.[14] Data from the four published trials are available.[15] Some forty carcinogenesis studies in rats or mice, reported in the Chemoprev.Database, also support that calcium could prevent intestinal cancer.[16]
See also
References
- ^ Marshall, W. J. (1995). Clinical Chemistry (3rd ed.). London: Mosby. ISBN 0-7234-2190-0.
- ^ Barrett KE, Barman SM, Boitano S, Brooks H, "Chapter 23. Hormonal Control of Calcium & Phosphate Metabolism & the Physiology of Bone" (Chapter). Barrett KE, Barman SM, Boitano S, Brooks H: Ganong's Review of Medical Physiology, 23e: http://www.accessmedicine.com/content.aspx?aID=5244785.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19779013, please use {{cite journal}} with
|pmid=19779013instead. - ^ http://www.vivo.colostate.edu/hbooks/pathphys/digestion/smallgut/absorb_minerals.html
- ^ Heaney RP (2000). "Calcium, dairy products and osteoporosis". J Am Coll Nutr. 19 (2 Suppl): 83S–99S. PMID 10759135.
- ^ López-López, A; Castellote-Bargalló, AI; Campoy-Folgoso, C; Rivero-Urgël, M; Tormo-Carnicé, R; Infante-Pina, D; López-Sabater, MC (2001). "The influence of dietary palmitic acid triacylglyceride position on the fatty acid, calcium and magnesium contents of at term newborn faeces". Early human development. 65: S83–94. doi:10.1016/S0378-3782(01)00210-9. PMID 11755039.
- ^ Van Breemen, C; Aaronson, P; Loutzenhiser, R (1978). "Sodium-calcium interactions in mammalian smooth muscle". Pharmacol Rev. 30 (2): 167–208. PMID 224400.
- ^ Graf, Ernst (1983). "Calcium binding to phytic acid". Journal of Agricultural and Food Chemistry. 31 (4): 851. doi:10.1021/jf00118a045.
- ^ Watts, P. S. (2009). "Effects of oxalic acid ingestion by sheep. II. Large doses to sheep on different diets". The Journal of Agricultural Science. 52 (2): 250. doi:10.1017/S0021859600036765.
- ^ Shultz, T D; Bollman, S; Kumar, R (June 1982). "Decreased intestinal calcium absorption in vivo and normal brush border membrane vesicle calcium uptake in cortisol-treated chickens: evidence for dissociation of calcium absorption from brush border vesicle uptake" (PDF). Proc Natl Acad Sci U S A. 79 (11): 3542–3546. doi:10.1073/pnas.79.11.3542. PMC 346457. PMID 6954501.
- ^ Prager, E. M.; Johnson, L. R. (2009). "Stress at the Synapse: Signal Transduction Mechanisms of Adrenal Steroids at Neuronal Membranes". Science Signaling. 2 (86): re5. doi:10.1126/scisignal.286re5. PMID 19724063.
- ^ Page 1094 (The Parathyroid Glands and Vitamin D) in: Walter F., PhD. Boron (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. p. 1300. ISBN 1-4160-2328-3.
- ^ Medical Physiology; Guyton, Saunders and Co. 1976pp.1062
- ^ Baron J, Beach M, Mandel J, van Stolk R, Haile R, Sandler R, Rothstein R, Summers R, Snover D, Beck G, Bond J, Greenberg E (1999). "Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group". N Engl J Med. 340 (2): 101–7. doi:10.1056/NEJM199901143400204. PMID 9887161.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Potency-Man
- ^ Calcium meta-analysis Colon Cancer chemoprevention systematic review
External links
- Calcium at Lab Tests Online
- Template:GeorgiaPhysiology
