Tert-Butyl alcohol: Difference between revisions
m Corrected category |
Natural occurence |
||
| Line 94: | Line 94: | ||
'''''tert''-Butyl alcohol''', or 2-methyl-2-propanol, is the simplest [[Alcohol#tertiary|tertiary alcohol]]. It is one of the four [[isomer]]s of [[butanol]]. ''tert''-Butanol is a clear liquid (or a colorless solid, depending on the ambient temperature) with a [[camphor]]-like odor. It is very soluble in water and miscible with [[ethanol]] and [[diethyl ether]]. It is unique among the isomers of butanol because it tends to be a solid at room temperature, with a melting point slightly above 25 °C. |
'''''tert''-Butyl alcohol''', or 2-methyl-2-propanol, is the simplest [[Alcohol#tertiary|tertiary alcohol]]. It is one of the four [[isomer]]s of [[butanol]]. ''tert''-Butanol is a clear liquid (or a colorless solid, depending on the ambient temperature) with a [[camphor]]-like odor. It is very soluble in water and miscible with [[ethanol]] and [[diethyl ether]]. It is unique among the isomers of butanol because it tends to be a solid at room temperature, with a melting point slightly above 25 °C. |
||
==Natural occurence== |
|||
''t''-Butyl alcohol have been identified but not quantified, in beer and chickpeas.<ref>http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+50</ref> |
|||
==Preparation== |
==Preparation== |
||
Revision as of 04:59, 5 March 2013
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Methylpropan-2-ol[1]
| |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 906698 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.809 | ||
| EC Number |
| ||
| 1833 | |||
| MeSH | tert-Butyl+Alcohol | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1120 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H10O | |||
| Molar mass | 74.123 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Camphorous | ||
| Density | 0.775 g/mL | ||
| miscible[2] | |||
| log P | 0.584 | ||
| Vapor pressure | 4.1 kPa (at 20 °C) | ||
Refractive index (nD)
|
1.387 | ||
| Thermochemistry | |||
Heat capacity (C)
|
215.37 J K−1 mol−1 | ||
Std molar
entropy (S⦵298) |
189.5 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−360.04–−358.36 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−2.64479–−2.64321 MJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H225, H319, H332, H335 | |||
| P210, P261, P305+P351+P338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 11 °C | ||
| Explosive limits | 2.4–8.0% | ||
| Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
tert-Butyl alcohol, or 2-methyl-2-propanol, is the simplest tertiary alcohol. It is one of the four isomers of butanol. tert-Butanol is a clear liquid (or a colorless solid, depending on the ambient temperature) with a camphor-like odor. It is very soluble in water and miscible with ethanol and diethyl ether. It is unique among the isomers of butanol because it tends to be a solid at room temperature, with a melting point slightly above 25 °C.
Natural occurence
t-Butyl alcohol have been identified but not quantified, in beer and chickpeas.[3]
Preparation
tert-Butyl alcohol is derived commercially from isobutane as a co-product of propylene oxide production. It can also be produced by the catalytic hydration of isobutylene, or by a Grignard reaction between acetone and methylmagnesium chloride.
Applications
tert-Butyl alcohol is used as a solvent, as a denaturant for ethanol, as an ingredient in paint removers, as an octane booster for gasoline, as an oxygenate gasoline additive, and as an intermediate in the synthesis of other chemical commodities such as MTBE, ETBE, TBHP, other flavors and perfumes.
Chemistry
As a tertiary alcohol, tert-butyl alcohol is more stable to oxidation and less reactive than the other isomers of butanol.
When tert-butyl alcohol is deprotonated with a strong base, the product is an alkoxide anion. In this case, it is tert-butoxide. For example, the commonly used organic reagent potassium tert-butoxide is prepared by refluxing dry tert-butanol with potassium metal.[4]
- K + tBuOH → tBuO−K+ + 1/2 H2
The tert-butoxide species is itself useful as a strong, non-nucleophilic base in organic chemistry. It is able to abstract acidic protons from the substrate molecule readily, but its steric bulk inhibits the group from participating in nucleophilic substitution, such as in a Williamson ether synthesis or an SN2 reaction.
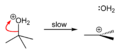
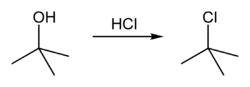
Conversion to alkyl halide
tert-Butyl alcohol reacts with hydrogen chloride to form tert-butyl chloride and water via an SN1 mechanism.
-
Step 1
-
Step 2
-
Step 3
The overall reaction, therefore, is:
Because tert-butyl alcohol is a tertiary alcohol, the relative stability of the tert-butyl carbocation in the Step 2 allows the SN1 mechanism to be followed. Primary alcohols generally undergo an SN2 mechanism because the relative stability of a primary carbocation intermediate is very low. The tertiary carbocation in this case is stabilized through hyperconjugation where the neighboring C–H sigma bonds donate electrons into the empty p-orbital of the carbocation.
References
- ^ a b c "tert-Butyl Alcohol - Compound Summary". PubChem Compound. National Center for Biotechnology Information. 2005-03-26. Retrieved 2012-05-19.
- ^ http://www.inchem.org/documents/icsc/icsc/eics0114.htm
- ^ http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+50
- ^ Johnson, W. S.; Schneider, W. P. (1950). "β-Carbethoxy-γ,γ-diphenylvinylacetic acid". Organic Syntheses. 30: 18; Collected Volumes, vol. 4, p. 132.
External links
- International Chemical Safety Card 0114
- NIOSH Pocket Guide to Chemical Hazards. "#0078". National Institute for Occupational Safety and Health (NIOSH).
- IPCS Environmental Health Criteria 65: Butanols: four isomers
- IPCS Health and Safety Guide 7: tert-Butanol