Tert-Amyl alcohol: Difference between revisions
No edit summary |
No edit summary |
||
| Line 81: | Line 81: | ||
2M2B has been used recreationally because of its lack of toxic aldehyde metabolites.<ref name='bluelight report'> {{cite web | url = http://www.bluelight.ru/vb/threads/439925-2-methyl-2-butanol-First-Time-Nice-Euphoric-Sedative-Few-Negatives | title = 2-methyl-2-butanol - First Time - Nice, Euphoric Sedative, Few Negatives | accessdate = 2013-01-13}}</ref><ref name='bluelight discussion'> {{cite web | url = http://www.bluelight.ru/vb/threads/490389-2-methyl-2-butanol-quot-Vodka-quot | title = 2-methyl-2-butanol "Vodka" | accessdate = 2013-01-13}}</ref> |
2M2B has been used recreationally because of its lack of toxic aldehyde metabolites.<ref name='bluelight report'> {{cite web | url = http://www.bluelight.ru/vb/threads/439925-2-methyl-2-butanol-First-Time-Nice-Euphoric-Sedative-Few-Negatives | title = 2-methyl-2-butanol - First Time - Nice, Euphoric Sedative, Few Negatives | accessdate = 2013-01-13}}</ref><ref name='bluelight discussion'> {{cite web | url = http://www.bluelight.ru/vb/threads/490389-2-methyl-2-butanol-quot-Vodka-quot | title = 2-methyl-2-butanol "Vodka" | accessdate = 2013-01-13}}</ref> |
||
==Occurrence |
==Occurrence== |
||
| ⚫ | |||
==Natural pollution sources== |
|||
2-Methyl-2-butanol has been identified as a volatile in [[cassava]]<ref>Dougan J et al; J Sci Food Agric 34: 874-84 (1983)</ref> and fried bacon(2) suggesting that it may be a naturally-occurring chemical(SRC).<ref>Ho CT et al; J Agric Food Chem 31: 336-42 (1983)</ref> |
|||
==Fusel alcohol== |
|||
| ⚫ | |||
==See also== |
==See also== |
||
Revision as of 02:32, 20 January 2013
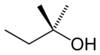
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methyl-2-butanol[1] | |
| Systematic IUPAC name
2-Methylbutan-2-ol[1] | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| 1361351 | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.827 |
| EC Number |
|
| KEGG | |
| MeSH | tert-amyl+alcohol |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1105 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12O | |
| Molar mass | 88.150 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Camphorous, peppermint |
| Density | 805 mg cm−3 |
| Melting point | −9 °C; 16 °F; 264 K |
| 120 g dm−3 | |
| log P | 1.095 |
| Vapor pressure | 1.6 kPa (at 20 °C) |
Refractive index (nD)
|
1.405 |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
229.3 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−380.0–−379.0 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−3.3036–−3.3026 MJ mol−1 |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H225, H315, H332, H335 | |
| P210, P261 | |
| NFPA 704 (fire diamond) | |
| Flash point | 19 °C |
| Explosive limits | 9% |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Methyl-2-butanol (2M2B), also known as tert-amyl alcohol (TAA), or amylene hydrate, is one of the isomers of amyl alcohol. It is a clear, colorless liquid with a strong odor of peppermint or camphor.[2][3] In humans it possesses sedative, hypnotic, and anticonvulsant effects similar to ethanol through ingestion or inhalation, and was previously used in medicine for this purpose.[4] It is active in doses of 2,000-4,000 mg, making it some 20 times more potent than regular ethanol.[5][6] Its hypnotic potency is between that of chloral hydrate and paraldehyde.[7] In humans, 2-methyl-2-butanol is metabolized primarily via gluconoridation and oxidation to 2,3-dihydroxy-2-methylbutane.[8] Overdose produces symptoms similar to alcohol poisoning and is a medical emergency.
2M2B has been used recreationally because of its lack of toxic aldehyde metabolites.[9][10]
Occurrence
Natural pollution sources
2-Methyl-2-butanol has been identified as a volatile in cassava[11] and fried bacon(2) suggesting that it may be a naturally-occurring chemical(SRC).[12]
Fusel alcohol
2M2B is a by-product of the fermentation of grain.[13]
See also
References
- ^ a b c d e f "tert-amyl alcohol - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 2011-12-13.
- ^ Coblentz, Virgil (1899). The Newer Remedies: A reference manual for physicians, pharmacists, and students (3rd ed.). Philadelphia: P. Blakiston's Son & Co. p. 18.
{{cite book}}: More than one of|at=and|page=specified (help) - ^ H. C. Wood & R. M. Smith, ed. (15 September 1887). "Amylene hydrate - a new hypnotic". Therapeutic Gazette - A monthly journal of physiological and clinical therapeutics. 3. Detroit MI and Philadelpia PA: G. S. Davis: 605–606.
- ^ Robert A. Lewis (1998). Lewis' Dictionary of Toxicology. CRC Press. p. 45. ISBN 1-56670-223-2.
- ^ Hans Brandenberger & Robert A. A. Maes, ed. (1997). Analytical Toxicology for Clinical, Forensic and Pharmaceutical Chemists. p. 401. ISBN 3-11-010731-7.
- ^ D. W. Yandell; et al. (1888). "Amylene hydrate, a new hypnotic". The American Practitioner and News. 5. Lousville KY: John P. Morton & Co: 88–89.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ F. A. Castle & C. Rice (1888). "Amylene and amylene hydrate". The American Druggist. 17 (3): 58–59.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 10367338 , please use {{cite journal}} with
|pmid= 10367338instead. - ^ "2-methyl-2-butanol - First Time - Nice, Euphoric Sedative, Few Negatives". Retrieved 2013-01-13.
- ^ "2-methyl-2-butanol "Vodka"". Retrieved 2013-01-13.
- ^ Dougan J et al; J Sci Food Agric 34: 874-84 (1983)
- ^ Ho CT et al; J Agric Food Chem 31: 336-42 (1983)
- ^ George Milbry Gould & R J E Scott. "The Practitioner's Medical Dictionary", 1910

