Electron transport chain: Difference between revisions
Corrected the number of water molecules produced in Complex IV and under “Electron acceptors” added a “citation needed” (since 2011).] |
Tobyfensome (talk | contribs) Page organisation majorly changed, lead article shortened, content minor changes, updated references, formatting issues. Tags: nowiki added Visual edit: Switched |
||
| Line 2: | Line 2: | ||
[[Image:Mitochondrial electron transport chain—Etc4.svg|thumb|300px|The electron transport chain in the mitochondrion is the site of [[oxidative phosphorylation]] in [[eukaryote]]s. The NADH and succinate generated in the [[citric acid cycle]] are oxidized, providing energy to power [[ATP synthase]].]] |
[[Image:Mitochondrial electron transport chain—Etc4.svg|thumb|300px|The electron transport chain in the mitochondrion is the site of [[oxidative phosphorylation]] in [[eukaryote]]s. The NADH and succinate generated in the [[citric acid cycle]] are oxidized, providing energy to power [[ATP synthase]].]] |
||
[[Image:Thylakoid membrane 3.svg|thumb|400px|Photosynthetic electron transport chain of the thylakoid membrane.]] |
[[Image:Thylakoid membrane 3.svg|thumb|400px|Photosynthetic electron transport chain of the thylakoid membrane.]] |
||
An '''electron transport chain''' (''' |
An '''electron transport chain''' ('''electron transport chain''') is a series of [[Protein complex|complexes]] that [[electron transfer|transfer]] [[electron]]s from [[electron donor]]s to [[electron acceptor]]s via [[redox]] (both reduction and oxidation occurring simultaneously) reactions, and couples this electron transfer with the transfer of [[proton]]s (H<sup>+</sup> ions) across a [[biological membrane|membrane]]. The electron transport chain is built up of [[peptide]]s, [[enzyme]]s (which are [[protein]]s or [[multiprotein complex|protein complexes]]), and other molecules. The flow of electrons through the electron transport chain is highly exergonic. The energy available, from the redox reactions, creates an [[electrochemical gradient|electrochemical proton gradient]] that drives the synthesis of [[adenosine triphosphate]] (ATP). In [[Cellular respiration|aerobic respiration]], the flow of electrons terminates with molecular [[oxygen]] being the final electron acceptor. In [[Anaerobic respiration|anerobic respiration]] other electron acceptors exist such as sulfate. |
||
In the electron transport chain, the redox reactions are driven by the [[Gibbs free energy]] state of the components. Gibbs free energy is related to a quantity called the redox potential. The complexes in the electron transport chain harvest the energy of the redox reactions, that occur when transferring electrons from a low redox potential to a higher redox potential, to create an electrochemical gradient. It is the electrochemical gradient created that drives the synthesis of ATP via coupling with [[Oxidative phosphorylation|oxidative phosphrylation]] with [[ATP synthase|ATP synthase.]]<ref>{{Cite journal|last=Anraku|first=Yasuhiro|date=1988-06|title=Bacterial Electron Transport Chains|url=http://www.annualreviews.org/doi/10.1146/annurev.bi.57.070188.000533|journal=Annual Review of Biochemistry|language=en|volume=57|issue=1|pages=101–132|doi=10.1146/annurev.bi.57.070188.000533|issn=0066-4154}}</ref> |
|||
Electron transport chains are used for extracting energy via redox reactions from [[sunlight]] in [[photosynthesis]] or, such as in the case of the oxidation of sugars, [[cellular respiration]]. In [[eukarya|eukaryotes]], an important electron transport chain is found in the [[inner mitochondrial membrane]] where it serves as the site of [[oxidative phosphorylation]] through the action of [[ATP synthase]]. It is also found in the [[thylakoid]] membrane of the [[chloroplast]] in photosynthetic eukaryotes. In [[bacteria]], the electron transport chain is located in their [[cell membrane]]. |
|||
The electron transport chain is found on the inner mitochrondrial membrane. This is the site of oxidative phosphorylation (the process of ATP synthesis by virtue of redox reactions) in eukaryotes. The energy that stored from the process of respiration in reduced compounds (such as NADH and FADH) is used by the electron transport chain to pump protons into the inter membrane space, generating the electrochemical gradient over the inner mitochrondrial membrane. In photosynthetic eukaryotes, the electron transport chain is found on the thlyakoid membrane. Here, light energy drives the reduction of components of the electron transport chain and therefor causes subsequent synthesis of ATP. In Bacteria, the electron transport chain can vary over species but in all cases the electron transport chain constitutes a set of redox reactions are coupled to the synthesis of ATP through the generation of an electrochemical gradient and oxidative phosprylation through ATP synthase.<ref>{{Cite journal|last=Kracke|first=Frauke|last2=Vassilev|first2=Igor|last3=Krömer|first3=Jens O.|date=2015|title=Microbial electron transport and energy conservation – the foundation for optimizing bioelectrochemical systems|url=https://www.frontiersin.org/articles/10.3389/fmicb.2015.00575/full|journal=Frontiers in Microbiology|language=English|volume=6|doi=10.3389/fmicb.2015.00575|issn=1664-302X|pmc=PMC4463002|pmid=26124754}}</ref> |
|||
In chloroplasts, light drives the [[Energy transformation|conversion]] of [[water]] to [[oxygen]] and [[Nicotinamide adenine dinucleotide phosphate|NADP<sup>+</sup>]] to NADPH with transfer of H<sup>+</sup> ions across [[Chloroplast#Structure|chloroplast membranes]]. In [[mitochondria]], it is the conversion of oxygen to water, NADH to NAD<sup>+</sup> and [[succinate]] to [[fumarate]] that are required to generate the proton gradient. |
|||
==Mitochrondrial electron transport chains== |
|||
Electron transport chains are major sites of premature electron leakage to oxygen, generating [[superoxide]] and potentially resulting in increased [[oxidative stress]]. |
|||
| ⚫ | Most [[eukaryotic]] cells have [[mitochondria]], which produce ATP from products of the [[citric acid cycle]], [[Fatty acid metabolism|fatty acid oxidation]], and [[Amino acid metabolism|amino acid oxidation]]. At the [[mitochondrial inner membrane]], electrons from [[NADH]] and [[FADH2|FADH<sub>2</sub>]]<nowiki/>pass through the electron transport chain to oxygen, which is reduced to water.<ref>{{Cite journal|last=Waldenström|first=Jan G.|date=2009-04-24|title=Biochemistry. By Lubert Stryer|url=http://dx.doi.org/10.1111/j.0954-6820.1975.tb19571.x|journal=Acta Medica Scandinavica|volume=198|issue=1-6|pages=436–436|doi=10.1111/j.0954-6820.1975.tb19571.x|issn=0001-6101}}</ref> The electron transport chain comprises an [[enzymatic]] series of electron donors and acceptors. Each [[electron donor]] will pass electrons to a more [[electronegative]] [[Electron acceptor|acceptor]], which in turn donates these electrons to another acceptor, a process that continues down the series until electrons are passed to oxygen, the most electronegative and terminal electron acceptor in the chain. Passage of electrons between donor and acceptor releases energy, which is used to generate a [[proton gradient]] across the mitochondrial membrane by [[proton pump|"pumping" protons]] into the intermembrane space, producing a thermodynamic state that has the potential to do work. This entire process is called [[oxidative phosphorylation]] since ADP is phosphrylated to ATP by using the electrochemical gradient established by the redox reactions of the electron transport chain. |
||
The electron transport chain consists of a spatially separated series of [[redox reaction]]s in which electrons are transferred from a donor molecule to an acceptor molecule. The underlying force driving these reactions is the [[Gibbs free energy]] of the reactants and products. The Gibbs free energy is the energy available ("free") to do work. Any reaction that decreases the overall Gibbs free energy of a system is thermodynamically spontaneous. |
|||
The function of the electron transport chain is to produce a transmembrane proton [[electrochemical gradient]] as a result of the redox reactions.<ref>{{cite book | last = Murray | first = Robert K. | authorlink = |author2=Daryl K. Granner |author3=Peter A. Mayes |author4=Victor W. Rodwell | title = Harper's Illustrated Biochemistry | publisher = Lange Medical Books/ MgGraw Hill | year = 2003 | location = New York, NY | pages = 96 | url = https://books.google.com/books?id=OJ7wAAAAMAAJ&dq=bibliogroup:%22HARPER%27S+BIOCHEMISTRY%22&ei=YwSjS8-OIYPYlQSJp93vBw&cd=2 | doi = | id = | isbn = 0-07-121766-5 }}</ref> If protons flow back through the membrane, they enable mechanical work, such as rotating bacterial [[flagella]]. [[ATP synthase]], an enzyme highly [[conserved sequence|conserved]] among all domains of life, converts this mechanical work into chemical energy by producing [[adenosine triphosphate|ATP]], which powers most cellular reactions.<ref>{{cite book | last = Karp | first = Gerald | authorlink = | title = Cell and Molecular Biology |edition=5th | publisher = John Wiley & Sons | year = 2008 | location = Hoboken, NJ | page = 194 | url = https://books.google.com/books?ei=IwGjS5T1MI2EkASTj_D6Bw&cd=5&id=-dBqAAAAMAAJ&dq=cell+molecular+biology+%22proton+gradient%22&q=%22translocation+of+protons+by+these+electron+transporting+complexes+establishes+the+proton+gradient%22#search_anchor | doi = | id = | isbn = 978-0-470-04217-5 }}</ref> In most organisms the majority of ATP is generated in electron transport chains. |
|||
==In mitochondria== |
|||
| ⚫ | Most [[eukaryotic]] cells have [[mitochondria]], which produce ATP from products of the [[citric acid cycle]], [[Fatty acid metabolism|fatty acid oxidation]], and [[Amino acid metabolism|amino acid oxidation]]. At the [[mitochondrial inner membrane]], electrons from [[NADH]] and [[FADH2]] |
||
A small percentage of electrons do not complete the whole series and instead directly leak to oxygen, resulting in the formation of the [[Reactive oxygen species|free-radical]] [[superoxide]], a highly reactive molecule that contributes to [[oxidative stress]] and has been implicated in a number of diseases and [[Senescence|aging]]. |
|||
===Mitochondrial redox carriers===<!-- This section is linked from [[Mitochondrion]] --> |
===Mitochondrial redox carriers===<!-- This section is linked from [[Mitochondrion]] --> |
||
Energy obtained through the transfer of electrons down the |
Energy obtained through the transfer of electrons down the electron transport chain is used to pump protons from the [[mitochondrial matrix]] into the intermembrane space, creating an electrochemical proton gradient ([[Oxidative phosphorylation#Chemiosmosis|ΔpH]]) across the inner mitochondrial membrane. This proton gradient is largely but not exclusively responsible for the mitochondrial [[membrane potential]] (ΔΨ<sub>M</sub>).<ref name="Zorova">{{cite journal |last1=Zorova |first1=LD |last2=Popkov |first2=VA |last3=Plotnikov |first3=EY |title=Mitochondrial membrane potential. |journal=Analytical biochemistry |date=1 July 2018 |volume=552 |pages=50-59 |doi=10.1016/j.ab.2017.07.009 |pmid=28711444}}</ref> It allows ATP synthase to use the flow of H<sup>+</sup> through the enzyme back into the matrix to generate ATP from [[adenosine diphosphate]] (ADP) and [[inorganic phosphate]]. Complex I (NADH coenzyme Q reductase; labeled I) accepts electrons from the [[Krebs cycle]] electron carrier [[Nicotinamide adenine dinucleotide|nicotinamide adenine dinucleotide (NADH)]], and passes them to coenzyme Q ([[ubiquinone]]; labeled Q), which also receives electrons from complex II ([[succinate dehydrogenase]]; labeled II). Q passes electrons to complex III ([[cytochrome bc1 complex|cytochrome bc<sub>1</sub> complex]]; labeled III), which passes them to [[cytochrome c|cytochrome ''c'']] (cyt ''c''). Cyt ''c'' passes electrons to Complex IV ([[cytochrome c oxidase|cytochrome ''c'' oxidase]]; labeled IV), which uses the electrons and hydrogen ions to reduce molecular oxygen to water. |
||
Four membrane-bound complexes have been identified in mitochondria. Each is an extremely complex transmembrane structure that is embedded in the inner membrane. |
Four membrane-bound complexes have been identified in mitochondria. Each is an extremely complex transmembrane structure that is embedded in the inner membrane. Three of them are proton pumps. The structures are electrically connected by lipid-soluble electron carriers and water-soluble electron carriers. The overall electron transport chain: |
||
'''NADH+H<sup>+</sup>''' →''' ''Complex I'' '''→ '''Q''' →''' ''Complex III'' '''→ '''cytochrome ''c'' '''→''' ''Complex IV'' ''' → '''H<sub>2</sub>O''' |
'''NADH+H<sup>+</sup>''' →''' ''Complex I'' '''→ '''Q''' →''' ''Complex III'' '''→ '''cytochrome ''c'' '''→''' ''Complex IV'' ''' → '''H<sub>2</sub>O''' |
||
| Line 32: | Line 24: | ||
====Complex I==== |
====Complex I==== |
||
{{Further|Respiratory complex I}} |
{{Further|Respiratory complex I}} |
||
In [[Respiratory complex I|Complex I]] (NADH ubiquinone oxireductase, Type I NADH dehydrogenase, or mitochondrial complex I; {{EC number|1.6.5.3}}), two electrons are removed from NADH and |
In [[Respiratory complex I|Complex I]] (NADH ubiquinone oxireductase, Type I NADH dehydrogenase, or mitochondrial complex I; {{EC number|1.6.5.3}}), two electrons are removed from NADH and transferred to a lipid-soluble carrier, ubiquinone (UQ). The reduced product, ubiquinol (UQH<sub>2</sub>), freely diffuses within the membrane, and Complex I translocates four protons (H<sup>+</sup>) across the membrane, thus producing a proton gradient. Complex I is one of the main sites at which premature electron leakage to oxygen occurs, thus being one of the main sites of production of superoxide.<ref name = "Lauren">Lauren, Biochemistry, Johnson/Cole, 2010, pp 598-611</ref> |
||
The pathway of electrons is as follows: |
The pathway of electrons is as follows: |
||
| Line 38: | Line 30: | ||
[[NADH]] is oxidized to NAD<sup>+</sup>, by reducing [[Flavin mononucleotide]] to FMNH<sub>2</sub> in one two-electron step. FMNH<sub>2</sub> is then oxidized in two one-electron steps, through a [[Ubiquinone#Chemical properties|semiquinone]] intermediate. Each electron thus transfers from the FMNH<sub>2</sub> to an [[Iron-sulfur cluster|Fe-S cluster]], from the Fe-S cluster to ubiquinone (Q). Transfer of the first electron results in the free-radical ([[Ubiquinone#Chemical properties|semiquinone]]) form of Q, and transfer of the second electron reduces the semiquinone form to the ubiquinol form, QH<sub>2</sub>. During this process, four protons are translocated from the mitochondrial matrix to the intermembrane space. |
[[NADH]] is oxidized to NAD<sup>+</sup>, by reducing [[Flavin mononucleotide]] to FMNH<sub>2</sub> in one two-electron step. FMNH<sub>2</sub> is then oxidized in two one-electron steps, through a [[Ubiquinone#Chemical properties|semiquinone]] intermediate. Each electron thus transfers from the FMNH<sub>2</sub> to an [[Iron-sulfur cluster|Fe-S cluster]], from the Fe-S cluster to ubiquinone (Q). Transfer of the first electron results in the free-radical ([[Ubiquinone#Chemical properties|semiquinone]]) form of Q, and transfer of the second electron reduces the semiquinone form to the ubiquinol form, QH<sub>2</sub>. During this process, four protons are translocated from the mitochondrial matrix to the intermembrane space. |
||
<ref name = "Garrett">Garrett & Grisham, Biochemistry, Brooks/Cole, 2010, pp 598-611</ref> As the electrons become continuously oxidized and reduced throughout the complex an electron current is produced along the 180 Angstrom width of the complex within the membrane. This current powers the active transport of four protons to the intermembrane space per two electrons from NADH.<ref>{{Cite book|title=biochemistry|last=Garret and Grisham|publisher=|year=2016|isbn=978-1-305-57720-6|location=University of Virginia|pages=687}}</ref> |
<ref name = "Garrett">Garrett & Grisham, Biochemistry, Brooks/Cole, 2010, pp 598-611</ref> As the electrons become continuously oxidized and reduced throughout the complex an electron current is produced along the 180 Angstrom width of the complex within the membrane. This current powers the active transport of four protons to the intermembrane space per two electrons from NADH.<ref>{{Cite book|title=biochemistry|last=Garret and Grisham|publisher=|year=2016|isbn=978-1-305-57720-6|location=University of Virginia|pages=687}}</ref> |
||
This complex is inhibited by Alkylguanides (Example : [[Guanethidine]]), [[Rotenone]], [[Barbiturate]]s, [[Chlorpromazine]], [[Piericidin A|Piericidin]]. |
|||
====Complex II==== |
====Complex II==== |
||
In ''Complex II'' ([[succinate dehydrogenase]] or succinate-CoQ reductase; {{EC number|1.3.5.1}}) additional electrons are delivered into the quinone pool (Q) originating from succinate and transferred (via [[Flavin adenine dinucleotide|flavin adenine dinucleotide (FAD)]]) to Q. Complex II consists of four protein subunits: |
In ''Complex II'' ([[succinate dehydrogenase]] or succinate-CoQ reductase; {{EC number|1.3.5.1}}) additional electrons are delivered into the quinone pool (Q) originating from succinate and transferred (via [[Flavin adenine dinucleotide|flavin adenine dinucleotide (FAD)]]) to Q. Complex II consists of four protein subunits: succinate dehydrogenase, (SDHA); succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial, (SDHB); succinate dehydrogenase complex subunit C, (SDHC) and succinate dehydrogenase complex, subunit D, (SDHD). Other electron donors (e.g., fatty acids and glycerol 3-phosphate) also direct electrons into Q (via FAD). Complex 2 is a parallel electron transport pathway to complex 1, but unlike complex 1, no protons are transported to the intermembrane space in this pathway. Therefore, the pathway through complex 2 contributes less energy to the overall electron transport chain process. |
||
This complex is inhibited by [https://pubchem.ncbi.nlm.nih.gov/compound/carboxin Carboxin]. |
|||
====Complex III==== |
====Complex III==== |
||
| Line 55: | Line 43: | ||
====Complex IV==== |
====Complex IV==== |
||
In ''Complex IV'' ([[cytochrome c oxidase|cytochrome ''c'' oxidase]]; {{EC number|1.9.3.1}}), sometimes called cytochrome AA3, four electrons are removed from four molecules of [[cytochrome c|cytochrome ''c'']] and transferred to molecular oxygen (O<sub>2</sub>), producing two molecules of water. At the same time, eight protons are removed from the mitochondrial matrix (although only four are translocated across the membrane), contributing to the proton gradient. The |
In ''Complex IV'' ([[cytochrome c oxidase|cytochrome ''c'' oxidase]]; {{EC number|1.9.3.1}}), sometimes called cytochrome AA3, four electrons are removed from four molecules of [[cytochrome c|cytochrome ''c'']] and transferred to molecular oxygen (O<sub>2</sub>), producing two molecules of water. The complex contains coordinated copper ions and several heme groups. At the same time, eight protons are removed from the mitochondrial matrix (although only four are translocated across the membrane), contributing to the proton gradient. The exact details of proton pumping in Complex IV are still under study.<ref name=":1">{{Cite book|last=Stryer.|url=http://worldcat.org/oclc/785100491|title=Biochemistry|publisher=toppan|oclc=785100491}}</ref> |
||
Cyanide blocks complex IV of the electron transport chain in mitochondria. If Complex IV is prohibited from performing its tasks, then the H+ ion gradient will not form within the mitochondrial membrane. This will then prohibit the production of ATP molecules. |
|||
===Coupling with oxidative phosphorylation=== |
===Coupling with oxidative phosphorylation=== |
||
[[File:ATP-Synthase.svg|thumb|240px|Depiction of [[ATP synthase]], the site of oxidative phosphorylation to generate ATP.]] |
[[File:ATP-Synthase.svg|thumb|240px|Depiction of [[ATP synthase]], the site of oxidative phosphorylation to generate ATP.]] |
||
The [[chemiosmosis|chemiosmotic coupling hypothesis]], proposed by [[Nobel Prize in Chemistry]] winner [[Peter D. Mitchell]], the electron transport chain and [[oxidative phosphorylation]] are coupled by a proton gradient across the inner mitochondrial membrane. The efflux of protons from the mitochondrial matrix creates an [[electrochemical gradient]] (proton gradient). This gradient is used by the F<sub>O</sub>F<sub>1</sub> [[ATP synthase]] complex to make ATP via oxidative phosphorylation. ATP synthase is sometimes described as ''Complex V'' of the electron transport chain.<ref>{{Cite journal|last=Jonckheere|first=An I.|last2=Smeitink|first2=Jan A. M.|last3=Rodenburg|first3=Richard J. T.|date=2017-03-10|title=Mitochondrial ATP synthase: architecture, function and pathology|journal=Journal of Inherited Metabolic Disease|volume=35|issue=2|pages=211–225|doi=10.1007/s10545-011-9382-9|issn=0141-8955| pmc=3278611 |pmid=21874297}}</ref> The F<sub>O</sub> component of [[ATP synthase]] acts as an [[ion channel]] that provides for a proton flux back into the mitochondrial matrix. It is composed of a, b and c subunits. Protons in the inter-membranous space of mitochondria first enters the ATP synthase complex through ''a'' subunit channel. Then protons move to the c subunits.<ref name=":0">{{Cite book|title=Biochemistry|last=Garrett|first=Reginald H.|last2=Grisham|first2=Charles M.|publisher=Cengage learning|year=2012|isbn=978-1-133-10629-6|edition=5th|pages=664}}</ref> The number of c subunits it has determines how many protons it will require to make the F<sub>O</sub> turn one full revolution. For example, in humans, there are 8 c subunits, thus 8 protons are required.<ref>{{Cite journal|last=Fillingame|first=Robert H|last2=Angevine|first2=Christine M|last3=Dmitriev|first3=Oleg Y|date=2003-11-27|title=Mechanics of coupling proton movements to c-ring rotation in ATP synthase|journal=FEBS Letters|language=en|volume=555|issue=1|pages=29–34|doi=10.1016/S0014-5793(03)01101-3|pmid=14630314|issn=1873-3468}}</ref> After ''c'' subunits, protons finally enters matrix using ''a'' subunit channel that opens into the mitochondrial matrix.<ref name=":0" /> This reflux releases [[Gibb's free energy|free energy]] produced during the generation of the oxidized forms of the electron carriers (NAD<sup>+</sup> and Q). The free energy is used to drive ATP synthesis, catalyzed by the F<sub>1</sub> component of the complex.<ref>{{Cite journal|last=Berg|first=Jeremy M.|last2=Tymoczko|first2=John L.|last3=Stryer|first3=Lubert|date=2002-01-01|title=A Proton Gradient Powers the Synthesis of ATP|url=https://www.ncbi.nlm.nih.gov/books/NBK22388/|language=en}}</ref> |
|||
<br> |
<br> |
||
Coupling with oxidative phosphorylation is a key step for ATP production. However, in specific cases, uncoupling the two processes may be biologically useful. The uncoupling protein, [[thermogenin]]—present in the inner mitochondrial membrane of [[brown adipose tissue]]—provides for an alternative flow of protons back to the inner mitochondrial matrix. Thyroxine is also a natural uncoupler. This alternative flow results in [[thermogenesis]] rather than ATP production.<ref>{{Cite journal|last=Cannon|first=Barbara|last2=Nedergaard|first2=Jan|date=2004-01-01|title=Brown Adipose Tissue: Function and Physiological Significance|url=http://physrev.physiology.org/content/84/1/277|journal=Physiological Reviews|language=en|volume=84|issue=1|pages=277–359|doi=10.1152/physrev.00015.2003|issn=0031-9333|pmid=14715917}}</ref> |
Coupling with oxidative phosphorylation is a key step for ATP production. However, in specific cases, uncoupling the two processes may be biologically useful. The uncoupling protein, [[thermogenin]]—present in the inner mitochondrial membrane of [[brown adipose tissue]]—provides for an alternative flow of protons back to the inner mitochondrial matrix. Thyroxine is also a natural uncoupler. This alternative flow results in [[thermogenesis]] rather than ATP production.<ref>{{Cite journal|last=Cannon|first=Barbara|last2=Nedergaard|first2=Jan|date=2004-01-01|title=Brown Adipose Tissue: Function and Physiological Significance|url=http://physrev.physiology.org/content/84/1/277|journal=Physiological Reviews|language=en|volume=84|issue=1|pages=277–359|doi=10.1152/physrev.00015.2003|issn=0031-9333|pmid=14715917}}</ref> |
||
=== |
=== Reverse electron flow === |
||
[[Reverse electron flow]], something called reverse electron transport, is the transfer of electrons through the electron transport chain through the reverse redox reactions. Usually requiring a signifcant amount of energy to be used, this can result in reducing the oxidised form of electron donors. For example, NAD+ can be reduced to NADH by complex I.<ref>{{Citation|last=Kim|first=Byung Hong|title=Introduction to bacterial physiology and metabolism|url=http://dx.doi.org/10.1017/cbo9780511790461.002|work=Bacterial Physiology and Metabolism|pages=1–6|publisher=Cambridge University Press|isbn=978-0-511-79046-1|access-date=2020-04-17|last2=Gadd|first2=Geoffrey Michael}}</ref> There are several factors that have been shown to induce reverse electron flow however more work needs to be done to confirm this. One such example is blockage of production of ATP by ATP synthase and therefor build up of a higher protein motive force inducing reverse electron flow.<ref>{{Cite journal|last=Mills|first=Evanna L.|last2=Kelly|first2=Beth|last3=Logan|first3=Angela|last4=Costa|first4=Ana S.H.|last5=Varma|first5=Mukund|last6=Bryant|first6=Clare E.|last7=Tourlomousis|first7=Panagiotis|last8=Däbritz|first8=J. Henry M.|last9=Gottlieb|first9=Eyal|last10=Latorre|first10=Isabel|last11=Corr|first11=Sinéad C.|date=2016-10|title=Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages|url=http://dx.doi.org/10.1016/j.cell.2016.08.064|journal=Cell|volume=167|issue=2|pages=457–470.e13|doi=10.1016/j.cell.2016.08.064|issn=0092-8674}}</ref> |
|||
In the mitochondrial electron transport chain electrons move from an electron donor (NADH or QH<sub>2</sub>) to a terminal electron acceptor (O<sub>2</sub>) via a series of redox reactions. These reactions are coupled to the creation of a proton gradient across the mitochondrial inner membrane. There are three proton pumps: ''I'', ''III'', and ''IV''. The resulting transmembrane proton gradient is used to make ATP via ATP synthase. |
|||
==Bacterial electron transport chains== |
|||
The reactions catalyzed by ''Complex I'' and ''Complex III'' work roughly at equilibrium. This means that these reactions are readily reversible, by increasing the concentration of the products relative to the concentration of the reactants (for example, by increasing the proton gradient). ATP synthase is also readily reversible. Thus ATP can be used to build a proton gradient, which in turn can be used to make NADH. This process of ''[[Reverse electron flow|reverse electron transport]]'' is important in many prokaryotic electron transport chains.<ref>{{Cite journal|last=Alberts|first=Bruce|last2=Johnson|first2=Alexander|last3=Lewis|first3=Julian|last4=Raff|first4=Martin|last5=Roberts|first5=Keith|last6=Walter|first6=Peter|date=2002-01-01|title=Electron-Transport Chains and Their Proton Pumps|url=https://www.ncbi.nlm.nih.gov/books/NBK26904/|language=en}}</ref> |
|||
| ⚫ | |||
==In bacteria== |
|||
| ⚫ | |||
'''NADH''' →''' ''Complex I'' '''→ '''Q''' →''' ''Complex III'' '''→ '''cytochrome ''c'' '''→''' ''Complex IV'' '''→ '''O<sub>2</sub>''' |
'''NADH''' →''' ''Complex I'' '''→ '''Q''' →''' ''Complex III'' '''→ '''cytochrome ''c'' '''→''' ''Complex IV'' '''→ '''O<sub>2</sub>''' |
||
where ''Complexes I, III'' and'' IV'' are proton pumps, while Q and cytochrome ''c'' are mobile electron carriers. |
where ''Complexes I, III'' and'' IV'' are proton pumps, while Q and cytochrome ''c'' are mobile electron carriers. The electron acceptor is molecular oxygen. |
||
In [[prokaryotes]] ([[bacteria]] and [[archaea]]) the situation is more complicated, because there are several different electron donors and several different electron acceptors. |
In [[prokaryotes]] ([[bacteria]] and [[archaea]]) the situation is more complicated, because there are several different electron donors and several different electron acceptors. The generalized electron transport chain in bacteria is: |
||
'''Donor''' '''Donor''' '''Donor''' |
'''Donor''' '''Donor''' '''Donor''' |
||
| Line 86: | Line 70: | ||
'''Acceptor''' '''Acceptor''' |
'''Acceptor''' '''Acceptor''' |
||
Note that electrons can enter the chain at three levels: at the level of a [[dehydrogenase]], at the level of the quinone pool, or at the level of a mobile [[cytochrome]] electron carrier. |
Note that electrons can enter the chain at three levels: at the level of a [[dehydrogenase]], at the level of the quinone pool, or at the level of a mobile [[cytochrome]] electron carrier. These levels correspond to successively more positive redox potentials, or to successively decreased potential differences relative to the terminal electron acceptor. In other words, they correspond to successively smaller Gibbs free energy changes for the overall redox reaction ''Donor → Acceptor''. |
||
Individual bacteria use multiple electron transport chains, often simultaneously. |
Individual bacteria use multiple electron transport chains, often simultaneously. Bacteria can use a number of different electron donors, a number of different dehydrogenases, a number of different oxidases and reductases, and a number of different electron acceptors. For example, ''E. coli'' (when growing aerobically using glucose as an energy source) uses two different NADH dehydrogenases and two different quinol oxidases, for a total of four different electron transport chains operating simultaneously. |
||
A common feature of all electron transport chains is the presence of a proton pump to create |
A common feature of all electron transport chains is the presence of a proton pump to create an electrochemical gradient over a membrane. Bacterial electron transport chains may contain as many as three proton pumps, like mitochondria, or they may contain only one or two. They always contain at least one proton pump. |
||
===Electron donors=== |
====Electron donors==== |
||
In the present day biosphere, the most common electron donors are organic molecules. |
In the present day biosphere, the most common electron donors are organic molecules. Organisms that use organic molecules as an electron source are called ''[[organotrophs]]''. Organotrophs (animals, fungi, protists) and ''[[phototrophs]]'' (plants and algae) constitute the vast majority of all familiar life forms. |
||
Some prokaryotes can |
Some prokaryotes can use inorganic matter as an energy source. Such an organism is called a ''[[lithotroph]]'' ("rock-eater"). Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, manganese oxide, and ferrous iron. Lithotrophs have been found growing in rock formations thousands of meters below the surface of Earth. Because of their volume of distribution, lithotrophs may actually outnumber organotrophs and phototrophs in our biosphere. |
||
The use of inorganic electron donors as an energy source is of particular interest in the study of evolution. |
The use of inorganic electron donors as an energy source is of particular interest in the study of evolution. This type of metabolism must logically have preceded the use of organic molecules as an energy source. |
||
=== |
====Complex I and II==== |
||
Bacteria can use a number of different electron donors. |
Bacteria can use a number of different electron donors. When organic matter is the energy source, the donor may be NADH or succinate, in which case electrons enter the electron transport chain via NADH dehydrogenase (similar to ''Complex I'' in mitochondria) or succinate dehydrogenase (similar to ''Complex II''). Other dehydrogenases may be used to process different energy sources: formate dehydrogenase, lactate dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase, H<sub>2</sub> dehydrogenase ([[hydrogenase]]), electron transport chain. Some dehydrogenases are also proton pumps; others funnel electrons into the quinone pool. Most dehydrogenases show induced expression in the bacterial cell in response to metabolic needs triggered by the environment in which the cells grow. In the case of lactate dehydrogenase in E.coli, the enzyme is used aerobically and in combination with other dehydrogenases. It is inducible and is expressed when there is high concentration of DL- lactate present in the cell.<ref>{{Cite journal|date=1965-03|url=http://dx.doi.org/10.1111/nyas.1965.118.issue-19|journal=Annals of the New York Academy of Sciences|volume=118|issue=19 Ionic Conduc|doi=10.1111/nyas.1965.118.issue-19|issn=0077-8923}}</ref> |
||
===Quinone carriers=== |
====Quinone carriers==== |
||
[[Quinone]]s are mobile, lipid-soluble carriers that shuttle electrons (and protons) between large, relatively immobile macromolecular complexes embedded in the membrane. |
[[Quinone]]s are mobile, lipid-soluble carriers that shuttle electrons (and protons) between large, relatively immobile macromolecular complexes embedded in the membrane. Bacteria use [[ubiquinone]] (Coenzyme Q, the same quinone that mitochondria use) and related quinones such as [[menaquinone]] (Vitamin K<sub>2</sub>). Archaea in the genus ''[[Sulfolobus]]'' use caldariellaquinone.<ref>{{EC number|1.3.5.1}}</ref> The use of different quinones is due to slighlty altered redox potentials. These changes in redox potential are caused by changes in structure of quinone. The Change in redox potentials of these quinones may be suited to changes in the electron acceptors or variations of redox potentials in bacterial complexes.<ref>{{Cite journal|last=Ingledew|first=W J|last2=Poole|first2=R K|date=1984|title=The respiratory chains of Escherichia coli.|url=http://dx.doi.org/10.1128/mmbr.48.3.222-271.1984|journal=Microbiological Reviews|volume=48|issue=3|pages=222–271|doi=10.1128/mmbr.48.3.222-271.1984|issn=0146-0749}}</ref> |
||
===Proton pumps=== |
====Proton pumps==== |
||
A ''[[proton pump]]'' is any process that creates a [[proton gradient]] across a membrane. |
A ''[[proton pump]]'' is any process that creates a [[proton gradient]] across a membrane. Protons can be physically moved across a membrane; this is seen in mitochondrial ''Complexes I'' and ''IV''. The same effect can be produced by moving electrons in the opposite direction. The result is the disappearance of a proton from the cytoplasm and the appearance of a proton in the periplasm. Mitochondrial ''Complex III'' uses this second type of proton pump, which is mediated by a quinone (the [[Q cycle]]). |
||
Some dehydrogenases are proton pumps; others are not. |
Some dehydrogenases are proton pumps; others are not. Most oxidases and reductases are proton pumps, but some are not. Cytochrome ''bc<sub>1</sub>'' is a proton pump found in many, but not all, bacteria (it is not found in ''E. coli''). As the name implies, bacterial ''bc<sub>1</sub>'' is similar to mitochondrial ''bc<sub>1</sub>'' (''Complex III''). |
||
| ⚫ | |||
Proton pumps are the heart of the electron transport process. They produce the transmembrane electrochemical gradient that enables ATP Synthase to synthesize ATP. |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | Electrons may enter an electron transport chain at the level of a mobile cytochrome or quinone carrier. For example, electrons from inorganic electron donors (nitrite, ferrous iron, electron transport chain.) enter the electron transport chain at the cytochrome level. When electrons enter at a redox level greater than NADH, the electron transport chain must operate in reverse to produce this necessary, higher-energy molecule. |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | Electrons may enter an electron transport chain at the level of a mobile cytochrome or quinone carrier. |
||
| ⚫ | When bacteria grow in [[Cellular respiration#Aerobic respiration|aerobic]] environments, the terminal electron acceptor (O<sub>2</sub>) is reduced to water by an enzyme called an ''oxidase''. When bacteria grow in [[Hypoxia (environmental)|anaerobic]] environments, the terminal electron acceptor is reduced by an enzyme called a reductase. In mitochondria the terminal membrane complex (''Complex IV'') is cytochrome oxidase. [[Cellular respiration#Aerobic respiration|Aerobic]] bacteria use a number of different terminal oxidases. For example, ''E. coli'' (a facultative anaerobe) does not have a cytochrome oxidase or a ''bc<sub>1</sub>'' complex. Under aerobic conditions, it uses two different terminal quinol oxidases (both proton pumps) to reduce oxygen to water. |
||
Bacterial Complex IV can be split into classes according to the molecules act as terminal electron acceptors. Class I oxidases are cytochrome oxidases and use oxygen as the terminal electron acceptor. Class II oxidases are Quinol oxidases and can use a variety of terminal electron acceptors. Both of these classes can be subdivided into catergories based on what redox active components they contain. E.g. Heme aa3 Class 1 terminal oxidases are much more efficient than Class 2 terminal oxidases<ref>{{Cite journal|last=Anraku|first=Yasuhiro|date=1988-06|title=Bacterial Electron Transport Chains|url=http://www.annualreviews.org/doi/10.1146/annurev.bi.57.070188.000533|journal=Annual Review of Biochemistry|language=en|volume=57|issue=1|pages=101–132|doi=10.1146/annurev.bi.57.070188.000533|issn=0066-4154}}</ref> |
|||
| ⚫ | |||
When bacteria grow in [[Cellular respiration#Aerobic respiration|aerobic]] environments, the terminal electron acceptor (O<sub>2</sub>) is reduced to water by an enzyme called an ''oxidase''. When bacteria grow in [[Hypoxia (environmental)|anaerobic]] environments, the terminal electron acceptor is reduced by an enzyme called a ''reductase''. |
|||
| ⚫ | [[Anaerobic organism|Anaerobic]] bacteria, which do not use oxygen as a terminal electron acceptor, have terminal reductases individualized to their terminal acceptor. For example, ''E. coli'' can use fumarate reductase, nitrate reductase, nitrite reductase, DMSO reductase, or trimethylamine-N-oxide reductase, depending on the availability of these acceptors in the environment. |
||
| ⚫ | In mitochondria the terminal membrane complex (''Complex IV'') is cytochrome oxidase. |
||
| ⚫ | |||
| ⚫ | [[Anaerobic organism|Anaerobic]] bacteria, which do not use oxygen as a terminal electron acceptor, have terminal reductases individualized to their terminal acceptor. |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | Just as there are a number of different electron donors (organic matter in organotrophs, inorganic matter in lithotrophs), there are a number of different electron acceptors, both organic and inorganic. In aerobic bacteria and facultative anaerobes if oxygen is available, it is invariably used as the terminal electron acceptor, because it generates the greatest Gibbs free energy change and produces the most energy.<ref name="Schmidt-Rohr 20"> Schmidt-Rohr, K. (2020). "Oxygen Is the High-Energy Molecule Powering Complex Multicellular Life: Fundamental Corrections to Traditional Bioenergetics” ''ACS Omega'' '''5''': 2221-2233. http://dx.doi.org/10.1021/acsomega.9b03352 </ref> |
||
| ⚫ | |||
| ⚫ | Just as there are a number of different electron donors (organic matter in organotrophs, inorganic matter in lithotrophs), there are a number of different |
||
In anaerobic environments, different electron acceptors are used, including nitrate, nitrite, ferric iron, sulfate, carbon dioxide, and small organic molecules such as fumarate. |
In anaerobic environments, different electron acceptors are used, including nitrate, nitrite, ferric iron, sulfate, carbon dioxide, and small organic molecules such as fumarate. |
||
Since electron transport chains are redox processes, they can be described as the sum of two redox pairs. For example, the mitochondrial electron transport chain can be described as the sum of the NAD<sup>+</sup>/NADH redox pair and the O<sub>2</sub>/H<sub>2</sub>O redox pair. NADH is the electron donor and O<sub>2</sub> is the electron acceptor. |
|||
Not every donor-acceptor combination is thermodynamically possible. The redox potential of the acceptor must be more positive than the redox potential of the donor. Furthermore, actual environmental conditions may be far different from ''standard'' conditions (1 molar concentrations, 1 atm partial pressures, pH = 7), which apply to ''standard'' redox potentials. For example, hydrogen-evolving bacteria grow at an ambient partial pressure of hydrogen gas of 10<sup>−4 </sup> atm. The associated redox reaction, which is thermodynamically favorable in nature, is thermodynamically impossible under "standard" conditions.{{Citation needed|date=April 2015}} |
|||
===Summary=== |
|||
Bacterial electron transport pathways are, in general, inducible. Depending on their environment, bacteria can synthesize different transmembrane complexes and produce different electron transport chains in their cell membranes. Bacteria select their electron transport chains from a DNA library containing multiple possible dehydrogenases, terminal oxidases and terminal reductases. The situation is often summarized by saying that electron transport chains in bacteria are ''branched'', ''modular'', and ''inducible''. |
|||
==Photosynthetic== |
==Photosynthetic== |
||
In [[oxidative phosphorylation]], electrons are transferred from a low-energy electron donor (e.g., NADH) to an acceptor (e.g., O<sub>2</sub>) through an electron transport chain. |
In [[oxidative phosphorylation]], electrons are transferred from a low-energy electron donor (e.g., NADH) to an acceptor (e.g., O<sub>2</sub>) through an electron transport chain. In [[photophosphorylation]], the energy of sunlight is used to ''create'' a high-energy electron donor which can subsequently reduce redox active components. These components are then coupled to ATP synthesis via proton translocation by the electron transport chain.<ref name=":1" /> |
||
| ⚫ | Photosynthetic electron transport chains, like the mitochondrial chain, can be considered as a special case of the bacterial systems. |
||
| ⚫ | Photosynthetic electron transport chains, like the mitochondrial chain, can be considered as a special case of the bacterial systems. They use mobile, lipid-soluble quinone carriers ([[phylloquinone]] and [[plastoquinone]]) and mobile, water-soluble carriers ([[cytochrome]]s, electron transport chain.). They also contain a [[proton pump]]. It is remarkable that the proton pump in ''all'' photosynthetic chains resembles mitochondrial ''Complex III''. The commonly-held theory of [[symbiogenesis]] believes that both organelles decended from bacteria. |
||
| ⚫ | |||
| ⚫ | |||
==Summary== |
|||
Electron transport chains are redox reactions that transfer electrons from an electron donor to an electron acceptor. The transfer of electrons is coupled to the translocation of protons across a membrane, producing a proton gradient. The proton gradient is used to produce useful work. |
|||
About 30 work units are produced per electron transport. |
|||
<!--== Notes == |
|||
<references group="nb" />--> |
|||
==See also== |
==See also== |
||
Revision as of 11:23, 17 April 2020


An electron transport chain (electron transport chain) is a series of complexes that transfer electrons from electron donors to electron acceptors via redox (both reduction and oxidation occurring simultaneously) reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. The electron transport chain is built up of peptides, enzymes (which are proteins or protein complexes), and other molecules. The flow of electrons through the electron transport chain is highly exergonic. The energy available, from the redox reactions, creates an electrochemical proton gradient that drives the synthesis of adenosine triphosphate (ATP). In aerobic respiration, the flow of electrons terminates with molecular oxygen being the final electron acceptor. In anerobic respiration other electron acceptors exist such as sulfate.
In the electron transport chain, the redox reactions are driven by the Gibbs free energy state of the components. Gibbs free energy is related to a quantity called the redox potential. The complexes in the electron transport chain harvest the energy of the redox reactions, that occur when transferring electrons from a low redox potential to a higher redox potential, to create an electrochemical gradient. It is the electrochemical gradient created that drives the synthesis of ATP via coupling with oxidative phosphrylation with ATP synthase.[1]
The electron transport chain is found on the inner mitochrondrial membrane. This is the site of oxidative phosphorylation (the process of ATP synthesis by virtue of redox reactions) in eukaryotes. The energy that stored from the process of respiration in reduced compounds (such as NADH and FADH) is used by the electron transport chain to pump protons into the inter membrane space, generating the electrochemical gradient over the inner mitochrondrial membrane. In photosynthetic eukaryotes, the electron transport chain is found on the thlyakoid membrane. Here, light energy drives the reduction of components of the electron transport chain and therefor causes subsequent synthesis of ATP. In Bacteria, the electron transport chain can vary over species but in all cases the electron transport chain constitutes a set of redox reactions are coupled to the synthesis of ATP through the generation of an electrochemical gradient and oxidative phosprylation through ATP synthase.[2]
Mitochrondrial electron transport chains
Most eukaryotic cells have mitochondria, which produce ATP from products of the citric acid cycle, fatty acid oxidation, and amino acid oxidation. At the mitochondrial inner membrane, electrons from NADH and FADH2pass through the electron transport chain to oxygen, which is reduced to water.[3] The electron transport chain comprises an enzymatic series of electron donors and acceptors. Each electron donor will pass electrons to a more electronegative acceptor, which in turn donates these electrons to another acceptor, a process that continues down the series until electrons are passed to oxygen, the most electronegative and terminal electron acceptor in the chain. Passage of electrons between donor and acceptor releases energy, which is used to generate a proton gradient across the mitochondrial membrane by "pumping" protons into the intermembrane space, producing a thermodynamic state that has the potential to do work. This entire process is called oxidative phosphorylation since ADP is phosphrylated to ATP by using the electrochemical gradient established by the redox reactions of the electron transport chain.
Mitochondrial redox carriers
Energy obtained through the transfer of electrons down the electron transport chain is used to pump protons from the mitochondrial matrix into the intermembrane space, creating an electrochemical proton gradient (ΔpH) across the inner mitochondrial membrane. This proton gradient is largely but not exclusively responsible for the mitochondrial membrane potential (ΔΨM).[4] It allows ATP synthase to use the flow of H+ through the enzyme back into the matrix to generate ATP from adenosine diphosphate (ADP) and inorganic phosphate. Complex I (NADH coenzyme Q reductase; labeled I) accepts electrons from the Krebs cycle electron carrier nicotinamide adenine dinucleotide (NADH), and passes them to coenzyme Q (ubiquinone; labeled Q), which also receives electrons from complex II (succinate dehydrogenase; labeled II). Q passes electrons to complex III (cytochrome bc1 complex; labeled III), which passes them to cytochrome c (cyt c). Cyt c passes electrons to Complex IV (cytochrome c oxidase; labeled IV), which uses the electrons and hydrogen ions to reduce molecular oxygen to water.
Four membrane-bound complexes have been identified in mitochondria. Each is an extremely complex transmembrane structure that is embedded in the inner membrane. Three of them are proton pumps. The structures are electrically connected by lipid-soluble electron carriers and water-soluble electron carriers. The overall electron transport chain:
NADH+H+ → Complex I → Q → Complex III → cytochrome c → Complex IV → H2O
↑
Complex II
↑
Succinate
Complex I
In Complex I (NADH ubiquinone oxireductase, Type I NADH dehydrogenase, or mitochondrial complex I; EC 1.6.5.3), two electrons are removed from NADH and transferred to a lipid-soluble carrier, ubiquinone (UQ). The reduced product, ubiquinol (UQH2), freely diffuses within the membrane, and Complex I translocates four protons (H+) across the membrane, thus producing a proton gradient. Complex I is one of the main sites at which premature electron leakage to oxygen occurs, thus being one of the main sites of production of superoxide.[5]
The pathway of electrons is as follows:
NADH is oxidized to NAD+, by reducing Flavin mononucleotide to FMNH2 in one two-electron step. FMNH2 is then oxidized in two one-electron steps, through a semiquinone intermediate. Each electron thus transfers from the FMNH2 to an Fe-S cluster, from the Fe-S cluster to ubiquinone (Q). Transfer of the first electron results in the free-radical (semiquinone) form of Q, and transfer of the second electron reduces the semiquinone form to the ubiquinol form, QH2. During this process, four protons are translocated from the mitochondrial matrix to the intermembrane space. [6] As the electrons become continuously oxidized and reduced throughout the complex an electron current is produced along the 180 Angstrom width of the complex within the membrane. This current powers the active transport of four protons to the intermembrane space per two electrons from NADH.[7]
Complex II
In Complex II (succinate dehydrogenase or succinate-CoQ reductase; EC 1.3.5.1) additional electrons are delivered into the quinone pool (Q) originating from succinate and transferred (via flavin adenine dinucleotide (FAD)) to Q. Complex II consists of four protein subunits: succinate dehydrogenase, (SDHA); succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial, (SDHB); succinate dehydrogenase complex subunit C, (SDHC) and succinate dehydrogenase complex, subunit D, (SDHD). Other electron donors (e.g., fatty acids and glycerol 3-phosphate) also direct electrons into Q (via FAD). Complex 2 is a parallel electron transport pathway to complex 1, but unlike complex 1, no protons are transported to the intermembrane space in this pathway. Therefore, the pathway through complex 2 contributes less energy to the overall electron transport chain process.
Complex III
In Complex III (cytochrome bc1 complex or CoQH2-cytochrome c reductase; EC 1.10.2.2), the Q-cycle contributes to the proton gradient by an asymmetric absorption/release of protons. Two electrons are removed from QH2 at the QO site and sequentially transferred to two molecules of cytochrome c, a water-soluble electron carrier located within the intermembrane space. The two other electrons sequentially pass across the protein to the Qi site where the quinone part of ubiquinone is reduced to quinol. A proton gradient is formed by one quinol () oxidations at the Qo site to form one quinone () at the Qi site. (In total, four protons are translocated: two protons reduce quinone to quinol and two protons are released from two ubiquinol molecules.)
When electron transfer is reduced (by a high membrane potential or respiratory inhibitors such as antimycin A), Complex III may leak electrons to molecular oxygen, resulting in superoxide formation.
This complex is inhibited by dimercaprol (British Antilewisite, BAL), Napthoquinone and Antimycin.
Complex IV
In Complex IV (cytochrome c oxidase; EC 1.9.3.1), sometimes called cytochrome AA3, four electrons are removed from four molecules of cytochrome c and transferred to molecular oxygen (O2), producing two molecules of water. The complex contains coordinated copper ions and several heme groups. At the same time, eight protons are removed from the mitochondrial matrix (although only four are translocated across the membrane), contributing to the proton gradient. The exact details of proton pumping in Complex IV are still under study.[8]
Coupling with oxidative phosphorylation
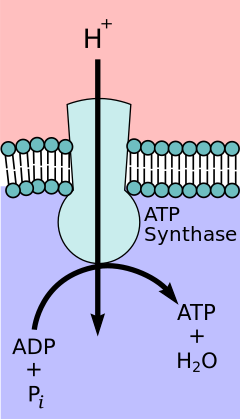
The chemiosmotic coupling hypothesis, proposed by Nobel Prize in Chemistry winner Peter D. Mitchell, the electron transport chain and oxidative phosphorylation are coupled by a proton gradient across the inner mitochondrial membrane. The efflux of protons from the mitochondrial matrix creates an electrochemical gradient (proton gradient). This gradient is used by the FOF1 ATP synthase complex to make ATP via oxidative phosphorylation. ATP synthase is sometimes described as Complex V of the electron transport chain.[9] The FO component of ATP synthase acts as an ion channel that provides for a proton flux back into the mitochondrial matrix. It is composed of a, b and c subunits. Protons in the inter-membranous space of mitochondria first enters the ATP synthase complex through a subunit channel. Then protons move to the c subunits.[10] The number of c subunits it has determines how many protons it will require to make the FO turn one full revolution. For example, in humans, there are 8 c subunits, thus 8 protons are required.[11] After c subunits, protons finally enters matrix using a subunit channel that opens into the mitochondrial matrix.[10] This reflux releases free energy produced during the generation of the oxidized forms of the electron carriers (NAD+ and Q). The free energy is used to drive ATP synthesis, catalyzed by the F1 component of the complex.[12]
Coupling with oxidative phosphorylation is a key step for ATP production. However, in specific cases, uncoupling the two processes may be biologically useful. The uncoupling protein, thermogenin—present in the inner mitochondrial membrane of brown adipose tissue—provides for an alternative flow of protons back to the inner mitochondrial matrix. Thyroxine is also a natural uncoupler. This alternative flow results in thermogenesis rather than ATP production.[13]
Reverse electron flow
Reverse electron flow, something called reverse electron transport, is the transfer of electrons through the electron transport chain through the reverse redox reactions. Usually requiring a signifcant amount of energy to be used, this can result in reducing the oxidised form of electron donors. For example, NAD+ can be reduced to NADH by complex I.[14] There are several factors that have been shown to induce reverse electron flow however more work needs to be done to confirm this. One such example is blockage of production of ATP by ATP synthase and therefor build up of a higher protein motive force inducing reverse electron flow.[15]
Bacterial electron transport chains
In eukaryotes, NADH is the most important electron donor. The associated electron transport chain is
NADH → Complex I → Q → Complex III → cytochrome c → Complex IV → O2 where Complexes I, III and IV are proton pumps, while Q and cytochrome c are mobile electron carriers. The electron acceptor is molecular oxygen.
In prokaryotes (bacteria and archaea) the situation is more complicated, because there are several different electron donors and several different electron acceptors. The generalized electron transport chain in bacteria is:
Donor Donor Donor
↓ ↓ ↓
dehydrogenase → quinone → bc1 → cytochrome
↓ ↓
oxidase(reductase) oxidase(reductase)
↓ ↓
Acceptor Acceptor
Note that electrons can enter the chain at three levels: at the level of a dehydrogenase, at the level of the quinone pool, or at the level of a mobile cytochrome electron carrier. These levels correspond to successively more positive redox potentials, or to successively decreased potential differences relative to the terminal electron acceptor. In other words, they correspond to successively smaller Gibbs free energy changes for the overall redox reaction Donor → Acceptor.
Individual bacteria use multiple electron transport chains, often simultaneously. Bacteria can use a number of different electron donors, a number of different dehydrogenases, a number of different oxidases and reductases, and a number of different electron acceptors. For example, E. coli (when growing aerobically using glucose as an energy source) uses two different NADH dehydrogenases and two different quinol oxidases, for a total of four different electron transport chains operating simultaneously.
A common feature of all electron transport chains is the presence of a proton pump to create an electrochemical gradient over a membrane. Bacterial electron transport chains may contain as many as three proton pumps, like mitochondria, or they may contain only one or two. They always contain at least one proton pump.
Electron donors
In the present day biosphere, the most common electron donors are organic molecules. Organisms that use organic molecules as an electron source are called organotrophs. Organotrophs (animals, fungi, protists) and phototrophs (plants and algae) constitute the vast majority of all familiar life forms.
Some prokaryotes can use inorganic matter as an energy source. Such an organism is called a lithotroph ("rock-eater"). Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, manganese oxide, and ferrous iron. Lithotrophs have been found growing in rock formations thousands of meters below the surface of Earth. Because of their volume of distribution, lithotrophs may actually outnumber organotrophs and phototrophs in our biosphere.
The use of inorganic electron donors as an energy source is of particular interest in the study of evolution. This type of metabolism must logically have preceded the use of organic molecules as an energy source.
Complex I and II
Bacteria can use a number of different electron donors. When organic matter is the energy source, the donor may be NADH or succinate, in which case electrons enter the electron transport chain via NADH dehydrogenase (similar to Complex I in mitochondria) or succinate dehydrogenase (similar to Complex II). Other dehydrogenases may be used to process different energy sources: formate dehydrogenase, lactate dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase, H2 dehydrogenase (hydrogenase), electron transport chain. Some dehydrogenases are also proton pumps; others funnel electrons into the quinone pool. Most dehydrogenases show induced expression in the bacterial cell in response to metabolic needs triggered by the environment in which the cells grow. In the case of lactate dehydrogenase in E.coli, the enzyme is used aerobically and in combination with other dehydrogenases. It is inducible and is expressed when there is high concentration of DL- lactate present in the cell.[16]
Quinone carriers
Quinones are mobile, lipid-soluble carriers that shuttle electrons (and protons) between large, relatively immobile macromolecular complexes embedded in the membrane. Bacteria use ubiquinone (Coenzyme Q, the same quinone that mitochondria use) and related quinones such as menaquinone (Vitamin K2). Archaea in the genus Sulfolobus use caldariellaquinone.[17] The use of different quinones is due to slighlty altered redox potentials. These changes in redox potential are caused by changes in structure of quinone. The Change in redox potentials of these quinones may be suited to changes in the electron acceptors or variations of redox potentials in bacterial complexes.[18]
Proton pumps
A proton pump is any process that creates a proton gradient across a membrane. Protons can be physically moved across a membrane; this is seen in mitochondrial Complexes I and IV. The same effect can be produced by moving electrons in the opposite direction. The result is the disappearance of a proton from the cytoplasm and the appearance of a proton in the periplasm. Mitochondrial Complex III uses this second type of proton pump, which is mediated by a quinone (the Q cycle).
Some dehydrogenases are proton pumps; others are not. Most oxidases and reductases are proton pumps, but some are not. Cytochrome bc1 is a proton pump found in many, but not all, bacteria (it is not found in E. coli). As the name implies, bacterial bc1 is similar to mitochondrial bc1 (Complex III).
Cytochrome electron carriers
Cytochromes are pigments that contain iron. They are found in two very different environments.
Some cytochromes are water-soluble carriers that shuttle electrons to and from large, immobile macromolecular structures imbedded in the membrane. The mobile cytochrome electron carrier in mitochondria is cytochrome c. Bacteria use a number of different mobile cytochrome electron carriers.
Other cytochromes are found within macromolecules such as Complex III and Complex IV. They also function as electron carriers, but in a very different, intramolecular, solid-state environment.
Electrons may enter an electron transport chain at the level of a mobile cytochrome or quinone carrier. For example, electrons from inorganic electron donors (nitrite, ferrous iron, electron transport chain.) enter the electron transport chain at the cytochrome level. When electrons enter at a redox level greater than NADH, the electron transport chain must operate in reverse to produce this necessary, higher-energy molecule.
Terminal oxidases and reductases
When bacteria grow in aerobic environments, the terminal electron acceptor (O2) is reduced to water by an enzyme called an oxidase. When bacteria grow in anaerobic environments, the terminal electron acceptor is reduced by an enzyme called a reductase. In mitochondria the terminal membrane complex (Complex IV) is cytochrome oxidase. Aerobic bacteria use a number of different terminal oxidases. For example, E. coli (a facultative anaerobe) does not have a cytochrome oxidase or a bc1 complex. Under aerobic conditions, it uses two different terminal quinol oxidases (both proton pumps) to reduce oxygen to water.
Bacterial Complex IV can be split into classes according to the molecules act as terminal electron acceptors. Class I oxidases are cytochrome oxidases and use oxygen as the terminal electron acceptor. Class II oxidases are Quinol oxidases and can use a variety of terminal electron acceptors. Both of these classes can be subdivided into catergories based on what redox active components they contain. E.g. Heme aa3 Class 1 terminal oxidases are much more efficient than Class 2 terminal oxidases[19]
Anaerobic bacteria, which do not use oxygen as a terminal electron acceptor, have terminal reductases individualized to their terminal acceptor. For example, E. coli can use fumarate reductase, nitrate reductase, nitrite reductase, DMSO reductase, or trimethylamine-N-oxide reductase, depending on the availability of these acceptors in the environment.
Most terminal oxidases and reductases are inducible. They are synthesized by the organism as needed, in response to specific environmental conditions.
Electron acceptors
Just as there are a number of different electron donors (organic matter in organotrophs, inorganic matter in lithotrophs), there are a number of different electron acceptors, both organic and inorganic. In aerobic bacteria and facultative anaerobes if oxygen is available, it is invariably used as the terminal electron acceptor, because it generates the greatest Gibbs free energy change and produces the most energy.[20]
In anaerobic environments, different electron acceptors are used, including nitrate, nitrite, ferric iron, sulfate, carbon dioxide, and small organic molecules such as fumarate.
Photosynthetic
In oxidative phosphorylation, electrons are transferred from a low-energy electron donor (e.g., NADH) to an acceptor (e.g., O2) through an electron transport chain. In photophosphorylation, the energy of sunlight is used to create a high-energy electron donor which can subsequently reduce redox active components. These components are then coupled to ATP synthesis via proton translocation by the electron transport chain.[8]
Photosynthetic electron transport chains, like the mitochondrial chain, can be considered as a special case of the bacterial systems. They use mobile, lipid-soluble quinone carriers (phylloquinone and plastoquinone) and mobile, water-soluble carriers (cytochromes, electron transport chain.). They also contain a proton pump. It is remarkable that the proton pump in all photosynthetic chains resembles mitochondrial Complex III. The commonly-held theory of symbiogenesis believes that both organelles decended from bacteria.
Photosynthetic electron transport chains are discussed in greater detail in the articles Light-dependent reaction. See also Photophosphorylation, Photosynthesis, and Photosynthetic reaction center. Although an outline is given by the diagram above.
See also
References
- ^ Anraku, Yasuhiro (1988-06). "Bacterial Electron Transport Chains". Annual Review of Biochemistry. 57 (1): 101–132. doi:10.1146/annurev.bi.57.070188.000533. ISSN 0066-4154.
{{cite journal}}: Check date values in:|date=(help) - ^ Kracke, Frauke; Vassilev, Igor; Krömer, Jens O. (2015). "Microbial electron transport and energy conservation – the foundation for optimizing bioelectrochemical systems". Frontiers in Microbiology. 6. doi:10.3389/fmicb.2015.00575. ISSN 1664-302X. PMC 4463002. PMID 26124754.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Waldenström, Jan G. (2009-04-24). "Biochemistry. By Lubert Stryer". Acta Medica Scandinavica. 198 (1–6): 436–436. doi:10.1111/j.0954-6820.1975.tb19571.x. ISSN 0001-6101.
- ^ Zorova, LD; Popkov, VA; Plotnikov, EY (1 July 2018). "Mitochondrial membrane potential". Analytical biochemistry. 552: 50–59. doi:10.1016/j.ab.2017.07.009. PMID 28711444.
- ^ Lauren, Biochemistry, Johnson/Cole, 2010, pp 598-611
- ^ Garrett & Grisham, Biochemistry, Brooks/Cole, 2010, pp 598-611
- ^ Garret and Grisham (2016). biochemistry. University of Virginia. p. 687. ISBN 978-1-305-57720-6.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ a b Stryer. Biochemistry. toppan. OCLC 785100491.
- ^ Jonckheere, An I.; Smeitink, Jan A. M.; Rodenburg, Richard J. T. (2017-03-10). "Mitochondrial ATP synthase: architecture, function and pathology". Journal of Inherited Metabolic Disease. 35 (2): 211–225. doi:10.1007/s10545-011-9382-9. ISSN 0141-8955. PMC 3278611. PMID 21874297.
- ^ a b Garrett, Reginald H.; Grisham, Charles M. (2012). Biochemistry (5th ed.). Cengage learning. p. 664. ISBN 978-1-133-10629-6.
- ^ Fillingame, Robert H; Angevine, Christine M; Dmitriev, Oleg Y (2003-11-27). "Mechanics of coupling proton movements to c-ring rotation in ATP synthase". FEBS Letters. 555 (1): 29–34. doi:10.1016/S0014-5793(03)01101-3. ISSN 1873-3468. PMID 14630314.
- ^ Berg, Jeremy M.; Tymoczko, John L.; Stryer, Lubert (2002-01-01). "A Proton Gradient Powers the Synthesis of ATP".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Cannon, Barbara; Nedergaard, Jan (2004-01-01). "Brown Adipose Tissue: Function and Physiological Significance". Physiological Reviews. 84 (1): 277–359. doi:10.1152/physrev.00015.2003. ISSN 0031-9333. PMID 14715917.
- ^ Kim, Byung Hong; Gadd, Geoffrey Michael, "Introduction to bacterial physiology and metabolism", Bacterial Physiology and Metabolism, Cambridge University Press, pp. 1–6, ISBN 978-0-511-79046-1, retrieved 2020-04-17
- ^ Mills, Evanna L.; Kelly, Beth; Logan, Angela; Costa, Ana S.H.; Varma, Mukund; Bryant, Clare E.; Tourlomousis, Panagiotis; Däbritz, J. Henry M.; Gottlieb, Eyal; Latorre, Isabel; Corr, Sinéad C. (2016-10). "Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages". Cell. 167 (2): 457–470.e13. doi:10.1016/j.cell.2016.08.064. ISSN 0092-8674.
{{cite journal}}: Check date values in:|date=(help) - ^ Annals of the New York Academy of Sciences. 118 (19 Ionic Conduc). 1965-03. doi:10.1111/nyas.1965.118.issue-19. ISSN 0077-8923 http://dx.doi.org/10.1111/nyas.1965.118.issue-19.
{{cite journal}}: Check date values in:|date=(help); Missing or empty|title=(help) - ^ EC 1.3.5.1
- ^ Ingledew, W J; Poole, R K (1984). "The respiratory chains of Escherichia coli". Microbiological Reviews. 48 (3): 222–271. doi:10.1128/mmbr.48.3.222-271.1984. ISSN 0146-0749.
- ^ Anraku, Yasuhiro (1988-06). "Bacterial Electron Transport Chains". Annual Review of Biochemistry. 57 (1): 101–132. doi:10.1146/annurev.bi.57.070188.000533. ISSN 0066-4154.
{{cite journal}}: Check date values in:|date=(help) - ^ Schmidt-Rohr, K. (2020). "Oxygen Is the High-Energy Molecule Powering Complex Multicellular Life: Fundamental Corrections to Traditional Bioenergetics” ACS Omega 5: 2221-2233. http://dx.doi.org/10.1021/acsomega.9b03352
- Fenchel T; King GM; Blackburn TH (September 2006). Bacterial Biogeochemistry: The Ecophysiology of Mineral Cycling (2nd ed.). Elsevier. ISBN 978-0-12-103455-9.
- Lengeler JW (January 1999). Drews G; Schlegel HG (eds.). Biology of the Prokaryotes. Blackwell Science. ISBN 978-0-632-05357-5.
- Nelson DL; Cox MM (April 2005). Lehninger Principles of Biochemistry (4th ed.). W. H. Freeman. ISBN 978-0-7167-4339-2.
- Nicholls DG; Ferguson SJ (July 2002). Bioenergetics 3. Academic Press. ISBN 978-0-12-518121-1.
- Stumm W; Morgan JJ (1996). Aquatic Chemistry (3rd ed.). John Wiley & Sons. ISBN 978-0-471-51185-4.
- Thauer RK; Jungermann K; Decker K (March 1977). "Energy conservation in chemotrophic anaerobic bacteria". Bacteriol Rev. 41 (1): 100–80. PMC 413997. PMID 860983.
- White D. (September 1999). The Physiology and Biochemistry of Prokaryotes (2nd ed.). Oxford University Press. ISBN 978-0-19-512579-5.
- Voet D; Voet JG (March 2004). Biochemistry. Vol. 28 (3rd ed.). John Wiley & Sons. pp. 124. ISBN 978-0-471-58651-7. PMID 10878303.
{{cite book}}:|journal=ignored (help) - Kim HS.; Patel, K; Muldoon-Jacobs, K; Bisht, KS; Aykin-Burns, N; Pennington, JD; Van Der Meer, R; Nguyen, P; et al. (January 2010). "SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress". Cancer Cell. 17 (1): 41–52. doi:10.1016/j.ccr.2009.11.023. PMC 3711519. PMID 20129246.
External links
- Electron+Transport+Chain+Complex+Proteins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Khan Academy, video lecture








