Triamcinolone: Difference between revisions
KolbertBot (talk | contribs) m Bot: HTTP→HTTPS (v485) |
Nøkkenbuer (talk | contribs) →Side effects: transferred modified text and citation originally added by 83.112.223.233 in Osteoarthritis § Medication §§ Intra-articular |
||
| Line 75: | Line 75: | ||
==Side effects== |
==Side effects== |
||
Side effects of triamcinolone include [[sore throat]], nosebleeds, increased coughing, headache, and runny nose.{{citation needed|date=November 2012}} White patches in the throat or nose indicate a serious side effect.{{clarify|date=March 2017}} Symptoms of an [[allergic reaction]] include rash, itch, swelling, severe dizziness, trouble breathing.<ref>{{cite web| title = Drugs and Treatments - Nasacort AQ Nasl - Patient Handout| publisher = [[WebMD]]| url = http://www.webmd.com/drugs/drug-16244-Nasacort+AQ.aspx?drugid=16244&drugname=Nasacort%20AQ| accessdate = 2008-03-24}}</ref> An additional side effect for women is a prolonged menstrual cycle. |
Side effects of triamcinolone include [[sore throat]], nosebleeds, increased coughing, headache, and runny nose.{{citation needed|date=November 2012}} White patches in the throat or nose indicate a serious side effect.{{clarify|date=March 2017}} Symptoms of an [[allergic reaction]] include rash, itch, swelling, severe dizziness, trouble breathing.<ref>{{cite web| title = Drugs and Treatments - Nasacort AQ Nasl - Patient Handout| publisher = [[WebMD]]| url = http://www.webmd.com/drugs/drug-16244-Nasacort+AQ.aspx?drugid=16244&drugname=Nasacort%20AQ| accessdate = 2008-03-24}}</ref> An additional side effect for women is a prolonged menstrual cycle. |
||
A 2018 study found that intra-articular triamcinolone is associated with an increase in intraocular pressure, which—if sustained for long periods of time or increases enough acutely—can cause permanent [[vision loss]].<ref>{{cite journal|last1=Taliaferro|first1=Kevin|last2=Crawford|first2=Alexander|last3=Jabara|first3=Justin|last4=Lynch|first4=Jonathan|last5=Jung|first5=Edward|last6=Zvirbulis|first6=Raimonds|last7=Banka|first7=Trevor|title=Intraocular Pressure Increases After Intraarticular Knee Injection With Triamcinolone but Not Hyaluronic Acid|url=https://www.researchgate.net/publication/323704421_Intraocular_Pressure_Increases_After_Intraarticular_Knee_Injection_With_Triamcinolone_but_Not_Hyaluronic_Acid|access-date=8 April 2018|url-access=registration|format=Epub abstract ahead of print|journal=[[Clinical Orthopaedics and Related Research]]|type=[[Levels of evidence|Level-II]] therapeutic study|publisher=[[Springer Science+Business Media]]|publication-date=9 March 2018|doi=10.1007/s11999.0000000000000261|issn=1528-1132|lccn= 53007647|oclc=01554937|pmid=29533245|dead-url=no|via=[[ResearchGate]]}}</ref> |
|||
==Chemistry== |
==Chemistry== |
||
Revision as of 00:15, 9 April 2018
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Kenalog Nasacort |
| Other names | Click show to see
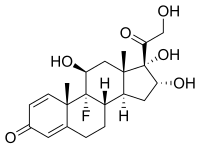
(8S,9R,10S,11S,13S,14S,16R,17S)-9-fluoro-11,16,17-trihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one; (1R,2S,10S,11S,13R,14S,15S,17S)-1-fluoro-13,14,17-trihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.02,7.011,15]heptadeca-3,6-dien-5-one |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Oral, topical, IM, intra-articular, intrasynovial |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 68% |
| Metabolism | Hepatic |
| Elimination half-life | 88 minutes |
| Excretion | Fecal and renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.290 |
| Chemical and physical data | |
| Formula | C21H27FO6 |
| Molar mass | 394.434 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Triamcinolone is an intermediate-acting synthetic glucocorticoid given orally, by injection, by inhalation, or as a topical ointment or cream.[1]
Uses
Triamcinolone is used to treat a number of different medical conditions, such as eczema, lichen sclerosus, psoriasis, arthritis, allergies, ulcerative colitis, lupus, sympathetic ophthalmia, temporal arteritis, uveitis, ocular inflammation, keloids, urushiol-induced contact dermatitis, aphthous ulcers (usually as triamcinolone acetonide), visualization during vitrectomy and the prevention of asthma attacks. It will not treat an asthma attack once it has already begun.[2][3][4] It has also been used off-label for macular degeneration.[5]
Prior to 2007 it was sold under the name Azmacort as a corticosteroid inhaler for asthma long-term care.
In 2010, TEVA and Perrigo launched the first generic inhalable triamcinolone.[6]
Triamcinolone is used to alleviate infection-induced eczema in fungal skin infections in the combination drug of econazole/triamcinolone.
The derivative triamcinolone acetonide is one of the ingredients of Ledermix, an endodontic (tooth's root canal) lotion used between sessions, and Sanofi sold it under the brand name Nasacort. Triamcinolone acetonide is also used as intra lesional steroid injection to treat keloids and hypertrophic scars.
According to Chang et al (2014), "Triamcinolone acetonide (TA) is classified as an S9 glucocorticoid in the 2014 Prohibited List published by the World Anti-Doping Agency, which caused it to be prohibited in-competition when administered orally, intravenously, intramuscularly or rectally".[7]
Forms
Different triamcinolone derivatives are available, including acetonide, benetonide, furetonide, hexacetonide and diacetate.
Triamcinolone acetonide is a more potent type of triamcinolone, being about eight times as effective as prednisone.
Side effects
Side effects of triamcinolone include sore throat, nosebleeds, increased coughing, headache, and runny nose.[citation needed] White patches in the throat or nose indicate a serious side effect.[clarification needed] Symptoms of an allergic reaction include rash, itch, swelling, severe dizziness, trouble breathing.[8] An additional side effect for women is a prolonged menstrual cycle.
A 2018 study found that intra-articular triamcinolone is associated with an increase in intraocular pressure, which—if sustained for long periods of time or increases enough acutely—can cause permanent vision loss.[9]
Chemistry
Triamcinolone is a synthetic pregnane corticosteroid and derivative of cortisol (hydrocortisone) and is also known as 1-dehydro-9α-fluoro-16α-hydroxyhydrocortisone or 9α-fluoro-16α-hydroxyprednisolone as well as 9α-fluoro-11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione.[10][11]
Society and culture
Brand names
Trade names for triamcinolone include Aristocort (Sandoz, now Novartis), Kenacort (Bristol-Myers Squibb), Kenalog (Bristol-Myers Squibb), Tricort (Cadila), Triaderm (Schering-Plough), Azmacort (KOS), Trilone, Volon A, Tristoject, Tricortone, Ratio-Triacomb, and Trianex.
See also
- Glucocorticoid (a chart comparing various glucocorticoids)
- Triamcinolone acetonide
References


- ^ Chrousos G, Pavlaki AN, Magiakou MA (2011). "Glucocorticoid Therapy and Adrenal Suppression". PMID 25905379.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Triamcinolone - Drugs.com
- ^ Triamcinolone Inhalation - Drugs.com
- ^ Alcon Receives FDA Approval of Triesence Injectable Triamcinolone Suspension for Use in Eye Surgery - Drugs.com
- ^ Age-Related Macular Degeneration (AMD) Treatment
- ^ Perrigo Announces Launch Of Generic Version Of Nasacort AQ - CBS Detroit
- ^ Chang, Chih-Wei; Huang, Tai-Yuan; Tseng, Yi-Chun; Chang-Chien, Guo-Ping; Lin, Su-Fan; Hsu, Mei-Chich (2014). "Positive doping results caused by the single-dose local injection of triamcinolone acetonide". Forensic Science International. 244: 1–6. doi:10.1016/j.forsciint.2014.07.024. PMID 25126738.
{{cite journal}}: Cite has empty unknown parameter:|1=(help) - ^ "Drugs and Treatments - Nasacort AQ Nasl - Patient Handout". WebMD. Retrieved 2008-03-24.
- ^ Taliaferro, Kevin; Crawford, Alexander; Jabara, Justin; Lynch, Jonathan; Jung, Edward; Zvirbulis, Raimonds; Banka, Trevor (9 March 2018). "Intraocular Pressure Increases After Intraarticular Knee Injection With Triamcinolone but Not Hyaluronic Acid" (Epub abstract ahead of print). Clinical Orthopaedics and Related Research (Level-II therapeutic study). Springer Science+Business Media. doi:10.1007/s11999.0000000000000261. ISSN 1528-1132. LCCN 53007647. OCLC 01554937. PMID 29533245. Retrieved 8 April 2018 – via ResearchGate.
{{cite journal}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - ^ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1228–. ISBN 978-1-4757-2085-3.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 1054–. ISBN 978-3-88763-075-1.
