Snakebite: Difference between revisions
DendroNaja (talk | contribs) |
DendroNaja (talk | contribs) |
||
| Line 137: | Line 137: | ||
==Snakes of particular concern== |
==Snakes of particular concern== |
||
===Ten most venomous snakes based on LD<sub>50</sub>=== |
|||
{{Collapse top|title=<span style="font-family:Palatino; font-size: 1.15em;">The most venomous land snakes by Ernst and Zug et al. (1996)</span>}} |
|||
{|class="wikitable" border="1" style= margin:0 1em 0.5em 1em;" |
|||
|+ Most venomous land snakes (Ernst and Zug ''et al.'' 1996)<ref name="ErZug">{{cite book |last= Ernst|first= Carl H.|last= Zug|first= George R.|title= Snakes in Question: The Smithsonian Answer Book |year= 1996|publisher= Smithsonian Institution Scholarly Press |location= Washington D.C., USA|isbn= 1-56098-648-4}}</ref> |
|||
|- |
|||
|'''Rank''' || '''Snake''' || '''Region''' || '''[[subcutaneous injection|SC]] {{LD50}}''' |
|||
|- |
|||
| 1 || [[Inland taipan]] || Australia || 0.01 mg/kg |
|||
|- |
|||
| 2 || [[Pseudonaja textilis|Eastern brown snake]] || Australia || 0.0365 mg/kg |
|||
|- |
|||
| 3 || [[Dubois' seasnake]] || Coral Sea, Arafura Sea, Timor Sea and Indian Ocean || 0.044 mg/kg |
|||
|- |
|||
| 4 || [[Black mamba]] || Sub-Saharan Africa || 0.05 mg/kg |
|||
|- |
|||
| 5 || [[Pelamis platura|Yellow bellied sea snake]] || Tropical oceanic waters || 0.067 mg/kg |
|||
|- |
|||
| 6 || [[Acalyptophis peronii|Peron's sea snake]] || Gulf of Siam, Strait of Taiwan, Coral sea islands, and other places || 0.079 mg/kg |
|||
|- |
|||
| 7 || [[Many-banded krait]] || Mainland China, Taiwan, Vietnam, Laos, Burma || 0.09 mg/kg |
|||
|- |
|||
| 8 || [[Coastal Taipan]] || Australia || 0.106 mg/kg |
|||
|- |
|||
| 9 || [[Black-banded sea krait]] || eastern coast of the Malay Peninsula and Brunei, and in Halmahera, Indonesia.. || 0.111 mg/kg |
|||
|- |
|||
| 10 || [[Beaked sea snake]] || Tropical Indo-Pacific || 0.1125 mg/kg |
|||
|} |
|||
{{collapse bottom}} |
|||
===Extremely Dangerous=== |
===Extremely Dangerous=== |
||
====Black mamba and Coastal taipan==== |
====Black mamba and Coastal taipan==== |
||
Revision as of 18:04, 24 October 2013
| Snakebite | |
|---|---|
| Specialty | Emergency medicine |
A snakebite is an injury caused by a bite from a snake, often resulting in puncture wounds inflicted by the animal's fangs and sometimes resulting in envenomation. Although the majority of snake species are non-venomous and typically kill their prey with constriction rather than venom, venomous snakes can be found on every continent except Antarctica.[1] Snakes often bite their prey as a method of hunting, but also for defensive purposes against predators. Since the physical appearance of snakes may differ, there is often no practical way to identify a species and professional medical attention should be sought.[2][3]
The outcome of snake bites depends on numerous factors, including the species of snake, the area of the body bitten, the amount of venom injected, and the health conditions of the person. Feelings of terror and panic are common after a snakebite and can produce a characteristic set of symptoms mediated by the autonomic nervous system, such as a racing heart and nausea.[4][5] Bites from non-venomous snakes can also cause injury, often due to lacerations caused by the snake's teeth, or from a resulting infection. A bite may also trigger an anaphylactic reaction, which is potentially fatal. First aid recommendations for bites depend on the snakes inhabiting the region, as effective treatments for bites inflicted by some species can be ineffective for others.
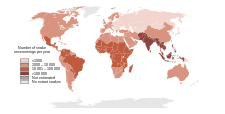
The number of fatalities attributed to snake bites varies greatly by geographical area. Although deaths are relatively rare in Australia, Europe and North America,[1][6][7] the morbidity and mortality associated with snake bites is a serious public health problem in many regions of the world, particularly in rural areas lacking medical facilities. Further, while South Asia, Southeast Asia, and sub-Saharan Africa report the highest number of bites, there is also a high incidence in the Neotropics and other equatorial and subtropical regions.[1][6][7] Each year tens of thousands of people die from snake bites,[1] yet the risk of being bitten can be lowered with preventive measures, such as wearing protective footwear and avoiding areas known to be inhabited by dangerous snakes.
Signs and symptoms

The most common symptoms of all snakebites are overwhelming fear, panic, and emotional instability, which may cause symptoms such as nausea and vomiting, diarrhea, vertigo, fainting, tachycardia, and cold, clammy skin.[4][5] Television, literature, and folklore are in part responsible for the hype surrounding snakebites, and people may have unwarranted thoughts of imminent death.
Dry snakebites, and those inflicted by a non-venomous species, can still cause severe injury. There are several reasons for this: a snakebite may become infected with the snake's saliva and fangs sometimes harboring pathogenic microbial organisms, including Clostridium tetani. Infection is often reported with viper bites whose fangs are capable of deep puncture wounds. Bites may cause anaphylaxis in certain people.
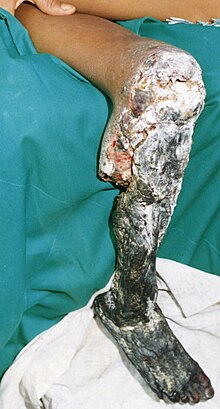
Most snakebites, whether by a venomous snake or not, will have some type of local effect. There is minor pain and redness in over 90% of cases, although this varies depending on the site.[4] Bites by vipers and some cobras may be extremely painful, with the local tissue sometimes becoming tender and severely swollen within 5 minutes.[7] This area may also bleed and blister and can eventually lead to tissue necrosis. Other common initial symptoms of pitviper and viper bites include lethargy, bleeding, weakness, nausea, and vomiting.[4][7] Symptoms may become more life-threatening over time, developing into hypotension, tachypnea, severe tachycardia, severe internal bleeding, altered sensorium, kidney failure, and respiratory failure.[4][7]
Interestingly, bites caused by the Mojave rattlesnake, kraits, coral snake, and the speckled rattlesnake reportedly cause little or no pain despite being serious injuries.[4] Those bitten may also describe a "rubbery," "minty," or "metallic" taste if bitten by certain species of rattlesnake.[4] Spitting cobras and rinkhalses can spit venom in a persons eyes. This results in immediate pain, ophthalmoparesis, and sometimes blindness.[11][12]
Some Australian elapids and most viper envenomations will cause coagulopathy, sometimes so severe that a person may bleed spontaneously from the mouth, nose, and even old, seemingly-healed wounds.[7] Internal organs may bleed, including the brain and intestines and will cause ecchymosis (bruising) of the skin.
Venom emitted from elapids, including sea snakes, kraits, cobras, king cobra, mambas, and many Australian species, contain toxins which attack the nervous system, causing neurotoxicity.[4][7][14] The person may present with strange disturbances to their vision, including blurriness. Paresthesia throughout the body, as well as difficulty in speaking and breathing, may be reported.[4] Nervous system problems will cause a huge array of symptoms, and those provided here are not exhaustive. If not treated immediately they may die from respiratory failure.
Venom emitted from some types of cobras, almost all vipers, some Australian elapids and some sea snakes causes necrosis of muscle tissue.[7] Muscle tissue will begin to die throughout the body, a condition known as rhabdomyolysis. Rhabdomyolysis can result in damage to the kidneys as a result of myoglobin accumulation in the renal tubules. This, coupled with hypotension, can lead to acute renal failure, and, if left untreated, eventually death.[7]
Pathophysiology
Since envenomation is completely voluntary, all venomous snakes are capable of biting without injecting venom into a person. Snakes may deliver such a "dry bite" rather than waste their venom on a creature too large for them to eat, a behavour called venom metering.[15] However, the percentage of dry bites varies between species: 80% of bites inflicted by sea snakes, which are normally timid, do not result in envenomation,[14] whereas only 25% of pitviper bites are dry.[4] Furthermore, some snake genera, such as rattlesnakes, significantly increase the amount of venom injected in defensive bites compared to predatory strikes.[16]
Some dry bites may also be the result of imprecise timing on the snake's part, as venom may be prematurely released before the fangs have penetrated the person.[15] Even without venom, some snakes, particularly large constrictors such as those belonging to the Boidae and Pythonidae families, can deliver damaging bites; large specimens often cause severe lacerations, or the snake itself pulls away, causing the flesh to be torn by the needle-sharp recurved teeth embedded in the person. While not as life-threatening as a bite from a venomous species, the bite can be at least temporarily debilitating and could lead to dangerous infections if improperly dealt with.
While most snakes must open their mouths before biting, African and Middle Eastern snakes belonging to the family Atractaspididae are able to fold their fangs to the side of their head without opening their mouth and jab a person.[17]
Snake venom
It has been suggested that snakes evolved the mechanisms necessary for venom formation and delivery sometime during the Miocene epoch.[18] During the mid-Tertiary, most snakes were large ambush predators belonging to the superfamily Henophidia, which use constriction to kill their prey. As open grasslands replaced forested areas in parts of the world, some snake families evolved to become smaller and thus more agile. However, subduing and killing prey became more difficult for the smaller snakes, leading to the evolution of snake venom.[18] Other research on Toxicofera, a hypothetical clade thought to be ancestral to most living reptiles, suggests an earlier time frame for the evolution of snake venom, possibly to the order of tens of millions of years, during the Late Cretaceous.[19]
Snake venom is produced in modified parotid glands normally responsible for secreting saliva. It is stored in structures called alveoli behind the animal's eyes, and ejected voluntarily through its hollow tubular fangs. Venom is composed of hundreds to thousands of different proteins and enzymes, all serving a variety of purposes, such as interfering with a prey's cardiac system or increasing tissue permeability so that venom is absorbed faster.
Venom in many snakes, such as pitvipers, affects virtually every organ system in the human body and can be a combination of many toxins, including cytotoxins, hemotoxins, neurotoxins, and myotoxins, allowing for an enormous variety of symptoms.[4][20] Earlier, the venom of a particular snake was considered to be one kind only i.e. either hemotoxic or neurotoxic, and this erroneous belief may still persist wherever the updated literature is hard to access. Although there is much known about the protein compositions of venoms from Asian and American snakes, comparatively little is known of Australian snakes.
The strength of venom differs markedly between species and even more so between families, as measured by median lethal dose (LD50) in mice. Subcutaneous LD50 varies by over 140-fold within elapids and by more than 100-fold in vipers. The amount of venom produced also differs among species, with the Gaboon viper able to potentially deliver from 450–600 milligrams of venom in a single bite, the most of any snake.[21] Opisthoglyphous colubrids have venom ranging from life-threatening (in the case of the boomslang) to barely noticeable (as in Tantilla).
Prevention

Snakes are most likely to bite when they feel threatened, are startled, are provoked, or have no means of escape when cornered. Encountering a snake is potentially dangerous and it is recommended to leave the vicinity. It is difficult to safely identify many snake species as appearances may vary dramatically.
Snakes are likely to approach residential areas when attracted by prey, such as rodents. Practising regular pest control can reduce the threat of snakes considerably. It is beneficial to know the species of snake that are common in local areas, or while travelling or hiking. Areas of the world such as Africa, Australia, the Neotropics, and southern Asia are inhabited by many highly dangerous species. Being wary of snake presence and ultimately avoiding it when known is strongly recommended.
When in the wilderness, treading heavily creates ground vibrations and noise, which will often cause snakes to flee from the area. However, this generally only applies to North America as some larger and more aggressive snakes in other parts of the world, such as king cobras[22] and black mambas,[23] will protect their territories. When dealing with direct encounters it is best to remain silent and motionless. If the snake has not yet fled it is important to step away slowly and cautiously.
The use of a flashlight when engaged in camping activities, such as gathering firewood at night, can be helpful. Snakes may also be unusually active during especially warm nights when ambient temperatures exceed 21 °C (70 °F). It is advised not to reach blindly into hollow logs, flip over large rocks, and enter old cabins or other potential snake hiding-places. When rock climbing, it is not safe to grab ledges or crevices without examining them first, as snakes are cold-blooded and often sunbathe atop rock ledges.
In the United States more than 40% of people bitten by snake intentionally put themselves in harm's way by attempting to capture wild snakes or by carelessly handling their dangerous pets—40% of that number had a blood alcohol level of 0.1% or more.[24]
It is also important to avoid snakes that appear to be dead, as some species will actually roll over on their backs and stick out their tongue to fool potential threats. A snake's detached head can immediately act by reflex and potentially bite. The induced bite can be just as severe as that of a live snake.[4][25] Dead snakes are also incapable of regulating the venom they inject, so a bite from a dead snake can often contain large amounts of venom.[26]
Treatment
It is not an easy task determining whether or not a bite by any species of snake is life-threatening. A bite by a North American copperhead on the ankle is usually a moderate injury to a healthy adult, but a bite to a child's abdomen or face by the same snake may be fatal. The outcome of all snakebites depends on a multitude of factors: the size, physical condition, and temperature of the snake, the age and physical condition of the person, the area and tissue bitten (e.g., foot, torso, vein or muscle), the amount of venom injected, the time it takes for the person to find treatment, and finally the quality of that treatment.[4][27]
Snake identification
Identification of the snake is important in planning treatment in certain areas of the world, but is not always possible. Ideally the dead snake would be brought in with the person, but in areas where snake bite is more common, local knowledge may be sufficient to recognize the snake. However, in regions where polyvalent antivenoms are available, such as North America, identification of snake is not a high priority item. Attempting to catch or kill the offending snake also puts one at risk for re-envenomation or creating a second person bitten, and generally is not recommended.
The three types of venomous snakes that cause the majority of major clinical problems are vipers, kraits, and cobras. Knowledge of what species are present locally can be crucial, as is knowledge of typical signs and symptoms of envenomation by each type of snake. A scoring system can be used to try to determine the biting snake based on clinical features,[28] but these scoring systems are extremely specific to particular geographical areas.
First aid
Snakebite first aid recommendations vary, in part because different snakes have different types of venom. Some have little local effect, but life-threatening systemic effects, in which case containing the venom in the region of the bite by pressure immobilization is desirable. Other venoms instigate localized tissue damage around the bitten area, and immobilization may increase the severity of the damage in this area, but also reduce the total area affected; whether this trade-off is desirable remains a point of controversy. Because snakes vary from one country to another, first aid methods also vary.
However, most first aid guidelines agree on the following:
- Protect the person and others from further bites. While identifying the species is desirable in certain regions, risking further bites or delaying proper medical treatment by attempting to capture or kill the snake is not recommended.
- Keep the person calm. Acute stress reaction increases blood flow and endangers the person. Panic is infectious and compromises judgment.
- Call for help to arrange for transport to the nearest hospital emergency room, where antivenom for snakes common to the area will often be available.
- Make sure to keep the bitten limb in a functional position and below the person's heart level so as to minimize blood returning to the heart and other organs of the body.
- Do not give the person anything to eat or drink. This is especially important with consumable alcohol, a known vasodilator which will speed up the absorption of venom. Do not administer stimulants or pain medications, unless specifically directed to do so by a physician.
- Remove any items or clothing which may constrict the bitten limb if it swells (rings, bracelets, watches, footwear, etc.)
- Keep the person as still as possible.
- Do not incise the bitten site.
Many organizations, including the American Medical Association and American Red Cross, recommend washing the bite with soap and water. Australian recommendations for snake bite treatment recommend against cleaning the wound. Traces of venom left on the skin/bandages from the strike can be used in combination with a snake bite identification kit to identify the species of snake. This speeds determination of which antivenom to administer in the emergency room.[29]
India developed a national snake-bite protocol in 2007 which includes advice to:[30]
- Reassure the patient. 70% of all snakebites are from non- venomous species. Only 50% of bites by venomous species actually envenomate the patient
- Immobilise in the same way as a fractured limb. Use bandages or cloth to hold the splints, not to block the blood supply or apply pressure. Do not apply any compression in the form of tight ligatures, they don’t work and can be dangerous!
- Get to Hospital Immediately. Traditional remedies have no proven benefit in treating snakebite.
- Tell the doctor of any systemic symptoms, such as droopiness of a body part, that manifest on the way to hospital.
Pressure immobilization

As of 2008, clinical evidence for pressure immobilization via the use of an elastic bandage is limited.[31] It is recommended for snakebite that have occurred in Australia (due to elapids which are neurotoxic).[32] It is not recommended for bites from non neurotoxic snakes such as found in North America and other regions of the world.[32][33] The British military recommends pressure immobilization in all cases where the type of snake is unknown.[34]
The object of pressure immobilization is to contain venom within a bitten limb and prevent it from moving through the lymphatic system to the vital organs. This therapy has two components: pressure to prevent lymphatic drainage, and immobilization of the bitten limb to prevent the pumping action of the skeletal muscles.
Antivenom
Until the advent of antivenom, bites from some species of snake were almost universally fatal.[35] Despite huge advances in emergency therapy, antivenom is often still the only effective treatment for envenomation. The first antivenom was developed in 1895 by French physician Albert Calmette for the treatment of Indian cobra bites. Antivenom is made by injecting a small amount of venom into an animal (usually a horse or sheep) to initiate an immune system response. The resulting antibodies are then harvested from the animal's blood.
Antivenom is injected into the person intravenously, and works by binding to and neutralizing venom enzymes. It cannot undo damage already caused by venom, so antivenom treatment should be sought as soon as possible. Modern antivenoms are usually polyvalent, making them effective against the venom of numerous snake species. Pharmaceutical companies which produce antivenom target their products against the species native to a particular area. Although some people may develop serious adverse reactions to antivenom, such as anaphylaxis, in emergency situations this is usually treatable and hence the benefit outweighs the potential consequences of not using antivenom.
Outmoded

The following treatments while once recommended are considered of no use or harmful including: tourniquets, incisions, suction, application of cold, and application of electricity.[33] Cases in which these treatments appear to work may be the result of dry bites.
- Application of a tourniquet to the bitten limb is generally not recommended. There is no convincing evidence that it is an effective first aid tool as ordinarily applied.[36] Tourniquets have been found to be completely ineffective in the treatment of Crotalus durissus bites,[37] but some positive results have been seen with properly applied tourniquets for cobra venom in the Philippines.[38] Uninformed tourniquet use is dangerous, since reducing or cutting off circulation can lead to gangrene, which can be fatal.[36] The use of a compression bandage is generally as effective, and much safer.
- Cutting open the bitten area, an action often taken prior to suction, is not recommended since it causes further damage and increases the risk of infection.
- Sucking out venom, either by mouth or with a pump, does not work and may harm the affected area directly.[39] Suction started after 3 minutes removes a clinically insignificant quantity—less than one thousandth of the venom injected—as shown in a human study.[40] In a study with pigs, suction not only caused no improvement but led to necrosis in the suctioned area.[41] Suctioning by mouth presents a risk of further poisoning through the mouth's mucous tissues.[42] The well-meaning family member or friend may also release bacteria into the persons wound, leading to infection.
- Immersion in warm water or sour milk, followed by the application of snake-stones (also known as la Pierre Noire), which are believed to draw off the poison in much the way a sponge soaks up water.
- Application of potassium permanganate.
- Use of electroshock therapy in animal tests has shown this treatment to be useless and potentially dangerous.[43][44][45][46]
In extreme cases, in remote areas, all of these misguided attempts at treatment have resulted in injuries far worse than an otherwise mild to moderate snakebite. In worst case scenarios, thoroughly constricting tourniquets have been applied to bitten limbs, completely shutting off blood flow to the area. By the time the person finally reached appropriate medical facilities their limbs had to be amputated.
Epidemiology
Most snakebites are caused by non-venomous snakes. Of the roughly 3,000 known species of snake found worldwide, only 15% are considered dangerous to humans.[1][4][47] Snakes are found on every continent except Antarctica.[1] The most diverse and widely distributed snake family, the colubrids, has approximately 700 venomous species,[48] but only five genera—boomslangs, twig snakes, keelback snakes, green snakes, and slender snakes—have caused human fatalities.[48]
Since reporting is not mandatory in many regions of the world,[1] snakebites often go unreported. Consequently, no accurate study has ever been conducted to determine the frequency of snakebites on the international level. However, some estimates put the number at 5.4 million snakebites, 2.5 million envenomings, resulting in perhaps 125,000 deaths.[1] Others estimate 1.2 to 5.5 million snakebites, 421,000 to 1.8 million envenomings, and 20,000 to 94,000 deaths.[1] Many people who survive bites nevertheless suffer from permanent tissue damage caused by venom, leading to disability.[7] Most snake envenomings and fatalities occur in South Asia, Southeast Asia, and sub-Saharan Africa, with India reporting the most snakebite deaths of any country.[1]
Worldwide, snakebites occur most frequently in the summer season when snakes are active and humans are outdoors.[1][49] Agricultural and tropical regions report more snakebites than anywhere else.[1][50] In the USA, those bitten are typically male and between 17 and 27 years of age.[4][49][51] Children and the elderly are the most likely to die.[4][27]
Snakes of particular concern
Ten most venomous snakes based on LD50
The most venomous land snakes by Ernst and Zug et al. (1996)
| ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Extremely Dangerous
Black mamba and Coastal taipan


Clinical mortality rate (often determined by measured toxicity on mice) is a commonly used indicator to determine the danger of any given venomous snake, but important too are its efficiency of venom delivery, its venom yield and its behavior when it encounters humans. Black mambas in particular are known to be high-strung, are the fastest snake species in the world, are highly aggressive, defensive and they are well known to have an irascible temperament.[22][53] Black mambas are also known to be the most accurate strikers, usually striking several times in quick succession. They are also known to have a 100% rate of envenomation. The probability of dry bites (no venom injected) in black mamba strikes is almost non-existent.[54][55] To date there has been no reported case of confirmed and medically treated black mamba bite in children.[56] Coastal taipans are also notoriously aggressive when cornered and will actively defend themselves vigorously.[57] Envenomation rate is very high, well over 80% of bites inject venom. Untreated black mamba (Dendroaspis polylepis) bites have a mortality rate of 100%.[22][54] Untreated coastal taipan (Oxyuranus scutellatus) bites have a mortality rate which ranges from 80-100%[58][57] Black mamba and coastal taipan bites require very rapid and vigorous antivenom therapy as they are almost always fatal. The venoms of both species are exceptionally quick acting and both can cause human fatality in as little as 15-30 minutes. Black mambas in particular have been known to cause death in as little as 20 minutes post-envenomation.[22][53] These two snake species are generally accepted to be the deadliest snakes in the world by most herpetologists.[59][60]
Highly Dangerous
Other species that are of particular concern are the common krait (Bungarus caeruleus), which is often considered to be the most dangerous Asian snake species. This species causes an estimated 10,000 fatalities throughout its range per year.[61] It is among the most venomous snakes in the world and has a 70-80% mortality rate. Venom yield per bite is 30 mg, while the lethal adult human dose is 2.5 mg.[62] Other krait species including the Malayan krait (Bungarus candidus), which ranked among the most venomous snakes in the world, is an extremely venomous and dangerous species.. In mice, the IV LD50 for this species is 0.1 mg/kg.[63] Envenomation rate among this species is very high and the untreated mortality is 70%, although even with antivenom and mechanical ventilation the mortality rate is at 50%.[64] The Many-banded krait (Bungarus multicinctus) is another krait species that is extremely venomous and dangerous. The venom of the many-banded krait consists of both pre- and postsynaptic neurotoxins (known as α-bungarotoxins and β-bungarotoxins, among others). The average venom yield from specimens kept on snake farms is about 4.6 mg[65]—18.4 mg per bite.[66] The venom is highly toxic with LD50 values of 0.09 mg/kg[66]—0.108 mg/kg[67][68] SC, 0.113 mg/kg IV and 0.08 mg/kg IP on mice.[68] Based on several LD50 studies, the many-banded krait is among the most venomous land snake in the world.[52] The Taiwan National Poison Control Center reports that the chief cause of deaths from snakebites during the past decade (2002-2012) was respiratory failure, 80% of which was caused by bites from the many-banded krait.[69]
The Inland taipan (Oxyuranus microlepidotus) is considered the most venomous snake in the world with a murine LD50 value of 0.025 mg/kg[70][71] and an average venom yield of 44 mg.[71] Bites from this species have a mortality rate of 80% if left untreated, although it is very rare for this species to bite. This species known to be a very shy, reclusive and a laid-back snake that will most always slither away from disturbance. It is not an aggressive species and rarely strikes.[71] The Eastern brown snake (Pseudonaja textilis) has a venom LD50 value of 0.053 mg, making it the second most venomous land snake in the world. Average venom yield is 2-6 mg according to (Meier and White, 1995). According to (Minton, 1974) average venom yield (dry weight) is between 5-10 mg.[72] This species is legendary for its bad temper, aggression, and for its speed. This species is responsible for more deaths every year in Australia than any other group of snakes.[73] The Common death adder (Acanthophis antarcticus) is a highly venomous snake species with a 50-60% untreated mortality rate.[74] It is also the fastest striking venomous snake in the world.[75] It lashes out with the quickest strike of any snake in the world. A death adder can go from a strike position, to strike and envenoming their prey, and back to strike position again, in less than 0.15 seconds.[75] The SC LD50 value is 0.4 mg/kg[76] and the venom yield per bite can range anywhere from 70-236 mg.[77] Unlike other snakes that flee from approaching humans crashing through the undergrowth, common death adders are more likely to sit tight and risk being stepped on, making them more dangerous to the unwary bushwalker. They are said to be reluctant to bite unless actually touched.[78] Tiger snakes (Notechis spp) are highly venomous. Their venoms possess potent neurotoxins, coagulants, haemolysins and myotoxins and the venom is quick-acting with rapid onset of breathing difficulties and paralysis. The untreated mortality rate from tiger snake bites is reported to be between 40 and 60%.[79] They are a major cause of snakebites and occasional snakebite deaths in Australia.[80]
The Saw-scaled viper (Echis carinatus) is small, but it's ill-temper, irritability, highly aggressive nature, loud hissing, and lethal venom potency make it very dangerous. This species is one of the fastest striking snakes in the world, and mortality rates for those bitten are very high. In India alone, the saw-scaled viper is responsible for an estimated 5,000 human fatalities annually.[61] However, because it ranges from Pakistan, India (in rocky regions of Maharastra, Rajasthan, Uttar Pradesh and Punjab), Sri Lanka, parts of the Middle East and Africa north of the equator,[81] is believed to cause more human fatalities every year than any other snake species.[82] In drier regions of the African continent, such as sahels and savannas, the saw-scaled vipers inflict up to 90% of all bites.[83] The rate of envenomation is over 80%.[84] The saw-scaled viper also produces a particularly painful bite. This species produces on the average of about 18 mg of dry venom by weight, with a recorded maximum of 72 mg. It may inject as much as 12 mg, whereas the lethal dose for an adult human is estimated to be only 5 mg.[85] Envenomation results in local symptoms as well as severe systemic symptoms that may prove fatal. Local symptoms include swelling and intense pain, which appear within minutes of a bite. In very bad cases the swelling may extend up the entire affected limb within 12–24 hours and blisters form on the skin.[86] Of the more dangerous systemic symptoms, hemorrhage and coagulation defects are the most striking. Hematemesis, melena, hemoptysis, hematuria and epistaxis also occur and may lead to hypovolemic shock. Almost all patients develop oliguria or anuria within a few hours to as late as 6 days post bite. In some cases, kidney dialysis is necessary due to acute renal failure (ARF), but this is not often caused by hypotension. It is more often the result of intravascular hemolysis, which occurs in about half of all cases. In other cases, ARF is often caused by disseminated intravascular coagulation.[86]
The Gaboon viper (Bitis gabonica), although generally docile and sluggish, have the longest fangs of any venomous snake and their venom glands are enormous and each bite produces the largest quantities of venom of any venomous snake. Yield is probably related to body weight, as opposed to milking interval.[87] Brown (1973) gives a venom yield range of 200–1000 mg (of dried venom),[67] A range of 200–600 mg for specimens 125–155 cm in length has also been reported.[87] Spawls and Branch (1995) state from 5 to 7 ml (450–600 mg) of venom may be injected in a single bite.[88] Based on how sensitive monkeys were to the venom, Whaler (1971) estimated 14 mg of venom would be enough to kill a human being: equivalent to 0.06 ml of venom, or 1/50 to 1/1000 of what can be obtained in a single milking. Marsh and Whaler (1984) wrote that 35 mg (1/30 of the average venom yield) would be enough to kill a man of 70 kilograms (150 lb).[87] A study by Marsh and Whaler (1984) reported a maximum yield of 9.7 ml of wet venom, which translated to 2400 mg of dried venom. They attached "alligator" clip electrodes to the angle of the open jaw of anesthetized specimens (length 133–136 cm, girth 23–25 cm, weight 1.3–3.4 kg), yielding 1.3–7.6 ml (mean 4.4 ml) of venom. Two to three electrical bursts within a space of five seconds apart were enough to empty the venom glands. The snakes used for the study were milked seven to 11 times over a 12-month period, during which they remained in good health and the potency of their venom remained the same.[87] In addition, Gaboon vipers produce the most painful bite of any venomous snake in the world. A bite causes very rapid and conspicuous swelling, intense pain, severe shock and local blistering. Other symptoms may include uncoordinated movements, defecation, urination, swelling of the tongue and eyelids, convulsions and unconsciousness.[87] Blistering, bruising and necrosis is often very extensive. There may be sudden hypotension, heart damage and dyspnoea.[89] The blood may become incoagulable with internal bleeding that may lead to haematuria and haematemesis.[88][89] Local tissue damage may require surgical excision and possibly amputation.[88] Healing may be slow and fatalities during the recovery period are not uncommon.[89]
Green mambas (Western, Eastern, and Jameson's) are all highly venomous snakes with bad tempers and a tendency to strike repeatedly with little provocation. The Western green mamba (Dendroaspis viridis) is highly venomous and aggressive with a LD50 of 0.7 mg/kg SC and the average venom yield per bite is approximately 100 mg. The mortality rate of untreated bites is unknown but is thought to be very high (>80%). The Eastern green mamba (Dendroaspis angusticeps) has an average venom yield per bite of 80 mg according to Engelmann and Obst (1981).[90] The subcutaneous LD50 for this species ranges from 0.40 mg/kg to 3.05 mg/kg depending on different toxicology studies, authority figures and estimates. The mortality rate of untreated bites is unknown but is thought to be very high (70-75%). The Jameson's mamba (Dendroaspis jamesoni) is known to be quite aggressive and defensive. The average venom yield per bite for this species is 80 mg, but some specimens may yield as much as 120 mg in a single bite. The SC LD50 for this species according to Brown (1973) is 1.0 mg/kg, while the IV LD50 is 0.8 mg/kg.[91] Envenomation by a Jameson's mamba can be deadly in as little as 30 to 120 minutes after being bitten, if proper medical treatment is not attained.[54] The mortality rate of untreated bites is not exactly known, but it's said to be very high (>80%).[92]
Other than the Saw-scaled viper and the common krait, there are two other species that are known to cause a high amount of human fatalities annually. The most well known are the Big Four. The Big Four are the four venomous snake species responsible for causing the most snake bite cases in South Asia (mostly in India). The Big Four snakes cause far more snakebites because they are much more abundant in highly-populated areas. They are the Indian cobra (Naja naja), common krait (Bungarus caeruleus), Russell's viper (Daboia russelii) and the Saw-scaled viper (Echis carinatus).[93] The Russell's viper is irritable, short-tempered and a very aggressive snake by nature and when it gets irritated it coils tightly, hisses, and strikes with a lightning speed. This species is responsible for more human fatalities in India than any other snakes. An estimated 25,000 fatalities are caused by this species annually.[61] The LD50 in mice, which is used as a possible indicator of snake venom toxicity, is as follows: 0.133 mg/kg intravenous, 0.40 mg/kg intraperitoneal, and about 0.75 mg/kg subcutaneous.[66] For most humans, a lethal dose is approximately 40–70 mg. However, the quantity of venom produced by individual specimens is considerable. Reported venom yields for adult specimens range from 130–250 mg to 150–250 mg to 21–268 mg. For 13 juveniles with an average length of 79 cm, the average venom yield was 8–79 mg (mean 45 mg).[87] The Russell's viper produces one of the most excruciatingly painful bites of all venomous snakes. Internal bleeding is common. Bruising, blistering and necrosis may appear relatively quickly aswell.[94] The Indian cobra is a moderately venomous species, but has a rapid-acting venom. In mice, the SC LD50 for this species is 0.80 mg/kg and the average venom yield per bite is between 169 and 250 mg.[67][95] Though it is responsible for many bites, only a small percentage are fatal if proper medical treatment and antivenom are given.[96] Mortality rate for untreated bite victims can vary from case to case, depending upon the quantity of venom delivered by the individual involved. According to one study, it is approximately 15–20%.[97] but in another study, with 1,224 bite cases, the mortality rate was only 6.5%.[67] Estimated fatalities as a result of this species is approximately 15,000 per year, but they are responsible for an estimated 100,000-150,000 non-fatal bites per year.[61]
Considerably Dangerous
The Terciopelo (Bothrops asper) has been described as excitable and unpredictable when disturbed. They can, and often will, move very quickly,[98] usually opting to flee from danger,[99] but are capable of suddenly reversing direction to vigorously defend themselves. Adult specimens, when cornered and fully alert, should be considered dangerous. In a review of bites from this species suffered by field biologists, Hardy (1994) referred to it as the "ultimate pit viper".[98] Venom yield (dry weight) averages 458 mg, with a maximum of 530 mg (Bolaños, 1984)[100] and an LD50 in mice of 2.844 mg/kg IP.[99] This species is an important cause of snakebite within its range. It is considered the most dangerous snake in Costa Rica, responsible for 46% of all bites and 30% of all hospitalized cases; before 1947, the fatality rate was 7%, but this has since declined to almost 0% (Bolaños, 1984), mostly due to the Clodomiro Picado Research Institute,[101] responsible for the production of antivenom. In the Colombian states of Antioquia and Chocó, it causes 50-70% of all snakebites, with a sequelae rate of 6% and a fatality rate of 5% (Otero et al., 1992). In the state of Lara, Venezuela, it is responsible for 78% of all envenomations and all snakebite fatalities (Dao-L., 1971). One of the reasons so many people are bitten is because of its association with human habitation and many bites actually occur indoors (Sasa & Vázquez, 2003). The Jararaca (Bothrops jararaca) is a species that is often abundant within its range, where it is an important cause of snakebite.[98] It is the best-known venomous snake in the wealthy and heavily populated areas of southeastern Brazil, where it was responsible for 52% (3,446 cases) of snakebites between 1902 and 1945, with a 0.7% mortality rate (25 deaths).[100] The average venom yield is 25–26 milligrams (0.39–0.40 gr) with a maximum of 300 milligrams (4.6 gr) of dried venom. The venom is only moderately toxic. In mice, the median lethal dose (LD50) is 1.2-1.3 mg/kg IV, 1.4 mg/kg IP and 3.0 mg/kg SC. For humans, the LD50 is estimated to be 210 milligrams (3.2 gr) subcutaneous.[67]
| Rank | Species | Venom LD50 |
|---|---|---|
| 1 | Congo water cobra (N. christyi) | 0.12 mg/kg[102] |
| 2 | Banded water cobra (N. annulata) | 0.143 mg/kg[102] |
| 3 | Philippine cobra (N. philippinensis) | 0.2 mg/kg[67] |
| 4 | Caspian cobra (N. oxiana) | 0.4 mg/kg[67] |
| 5 | Samar cobra (N. samarensis) | 0.46 mg/kg[103] |
| 6 | Equatorial spitting cobra (N. sumatrana) | 0.50 mg/kg[104] |
| 7 | Chinese cobra (N. atra) | 0.53 mg/kg[105] |
| 8 | Forest cobra (N. melanoleuca) | 0.6 mg/kg[67] |
| 9 | Cape cobra (N. nivea) | 0.72 mg/kg[67] |
| 10 | Indian cobra (N. naja) | 0.80 mg/kg[67][95] |
|} The cobras (Naja spp) are a medically important group of snakes. The Forest cobra (Naja melanoleuca) is the largest true cobra of the Naja species and is a very bad-tempered, aggressive, and irritable snake when cornered or molested. The species has a murine IP LD50 value of 0.324 mg/kg, while the IV LD50 value is 0.6 mg/kg, while the average venom yield is massive: 500 mg.[70] The forest cobra is one of the least frequent causes of snake bite among the African cobras, this is largely due to its forest-dwelling habits. Although it the largest of the Naja cobras, the venom is considered moderately toxic. If the snake becomes cornered or is agitated, it can quickly attack the aggressor, and if a large amount of venom is injected, a rapidly fatal outcome is possible. Clinical experience with forest cobras has been very sparse, and few recorded bites have been documented. However, in 2008, around the area of Friguiagbé in Guinea, there were 375 bites attributed to the forest cobra and of those 79 were fatal. Most of the fatal bites were patients who received no medical treatment.[106] Deaths from respiratory failure have been reported, but most victims will survive if prompt administration of antivenom is undertaken as soon as clinical signs of envenomation have been noted.[107] The Chinese cobra (Naja atra) is a highly venomous member of the true cobras (genus Naja). Its venom consists mainly of postsynaptic neurotoxins and cardiotoxins. Four cardiotoxin-analogues I, II, III, and IV, account for about 54% of the dry weight of the crude venom and have cytotoxic properties.[108] The LD50 values of its venom in mice are 0.29 mg/kg IV,[90] and a range of 0.29 mg/kg - 0.67 mg/kg[109][90] SC. The average venom yield from a snake of this species kept at a snake farm was about 250.8 mg (80 mg dry weight).[109] According to Minton (1974), this cobra has a venom yield range of 150 to 200 mg (dry weight).[110] Brown listed a venom yield of 184 mg (dry weight).[67] It is one of the most prevalent venomous snakes in mainland China and Taiwan, which has caused many snakebite incidents to humans. The most medically important species of snake in Central Asia is the Caspian cobra (Naja oxiana). The venom is primarily a potent neurotoxin but it also has some cytotoxic activity (tissue-death, necrosis).[111] Two forms of "cytotoxin II" (cardiotoxin) were found in the venom of this species.[112] The Caspian cobra is one of the most venomous species of cobra in the world. It has a subcutaneous LD50 value is 0.4 mg/kg and as a fairly large cobra, it can yield large volumes of venom in a single bite. Average venom yield per bite for this species is between 75 and 125 mg (dry weight), but it may yield up to 300 mg (dry weight). The lethal human dose is approximately 50 mg.[67] The bite of this species may cause severe pain and swelling, along with severe neurotoxicity. Weakness, drowsiness, ataxia, hypotension, and paralysis of throat and limbs may appear in less than one hour after the bite. Without medical treatment, symptoms rapidly worsen and death can occur rapidly after a bite due to respiratory failure. An adult woman bitten by this species in northwestern Pakistan suffered severe neurotoxicity and died while en route to the closest hospital nearly 50 minutes after envenomation. Between 1979 and 1987, 136 confirmed bites were attributed to this species in the former Soviet Union. Of the 136, 121 received antivenom, and only four died. Of the 15 who did not receive antivenom, 11 died. Death is uncommon when treated promptly with antivenom, however, the untreated mortality rate for this species is 70-75%, which is the highest among all cobra species of the genus Naja.[113] The African Cape cobra (Naja nivea) is is often regarded as one of the most dangerous species of cobra in all of Africa, by virtue of its potent venom and frequent occurrence in populated areas.[114] The Cape cobras venom is made up of potent postsynaptic neurotoxins and might also contain cardiotoxins,[92] that affect the respiratory system, nervous system, and the heart. The murine SC LD50 for this species' venom is 0.72 mg/kg, while the IV and IP LD50 values are 0.4 mg/kg and 0.6 mg/kg, respectively.[67] The average venom yield per bite is 100 to 150 mg according to Minton. Due to its highly toxic venom, its unrelenting aggressive deamnor, and its propensity to live near human habitats, the Cape cobra is clearly a very dangerous snakes. Envenomation by this species should be considered a serious medical emenergency. Provided that the diagnosis of clinical envenomation is made as early, antivenom therapy is instituted early, and ventilatory support is provided, the patient should do well. Mortality rates among those treated is very low (<10), while untreated mortality rates are estimated to be as high as 30-40%.[115] This species responsible for many bites in South Africa.[106] The Asian Monocled cobra (Naja kaouthia) is a medically important species as it is responsible for a considerable amount of bites throughout its range. The major toxic components in the Monocled cobras venom are postsynaptic neurotoxins, which block the nerve transmission by binding specifically to the nicotinic acetylcholine receptor, leading to flaccid paralysis and even death by respiratory failure. The major α-neurotoxin in Naja kaouthia venom is a long neurotoxin, α-cobratoxin; the minor α-neurotoxin is different from cobrotoxin in one residue.[116] The neurotoxins of this particular species are weak.[117] The venom of this species also contains myotoxins and cardiotoxins.[118][119] The median lethal dose (LD50) is 0.28-0.33 mg per gram of mouse body weight.[120] In case of IV the LD50 is 0.373 mg/kg, and 0.225 mg/kg in case of IP. The average venom yield per bite is approximately 263 mg (dry weight).[67] The monocled cobra causes the highest fatality due to snake venom poisoning in Thailand.[121] Envenomation usually presents predominantly with extensive local necrosis and systemic manifestations to a lesser degree. Drowsiness, neurological and neuromuscular symptoms will usually manifest earliest; hypotension, flushing of the face, warm skin, and pain around bite site typically manifest within one to four hours following the bite; paralysis, ventilatory failure or death could ensue rapidly, possibly as early as 60 minutes in very severe cases of envenomation. However, the presence of fang marks does not always imply that envenomation actually occurred.[54] The Egyptian cobra (Naja haje) is another species of cobra which causes a significant amount of bites and human fatalities throughout its range. The venom of the Egyptian cobra consists mainly of neurotoxins and cytotoxins.[122] The average venom yield is 175 to 300 mg in a single bite, and the murine subcutaneous LD50 value is 1.15 mg/kg. This species has large fangs and can produce large quantities of venom. Envenomation by this snake is a very serious medical emergency.[67]
The water cobras found in central and western Africa are the most venomous cobra species (Naja) in the world. These species were formerly under the genus Boulengerina. The Banded water cobra (Naja annulata) and the Congo water cobra (Naja christyi) are dangerously venomous. The banded water cobra has one subspecies which is known as Storms water cobra (Naja annulata stormsi). Their venoms are extremely potent neurotoxins. A toxicological study listed the intraperitoneal (IP) LD50 of N. annulata at 0.143 mg/kg.[102] Brown (1973) listed the intravenous LD50 for N. a. annulata at 0.2 mg/kg.[67] The same study listed the intraperitoneal (IP) LD50 of N. christyi at 0.12 mg/kg. The venoms of these little-known elapids have the lowest intraperitoneal LD50 of any Naja species studied thus far and have high concentrations of potent postsynaptic neurotoxins.[102] Serious and dangerous envenomation can result from a bite from either of these snakes. There is at least one case of human envenomation caused by the Congo water cobra (N. christyi). Symptoms of the envenomation were mild. There is no specific antivenom currently produced for either of these two species.[123]
The Black desert cobra (Walterinnesia aegyptia) is a highly venomous snake found in the Middle East. The subcutaneous LD50 for the venom of the Desert cobra is 0.4 mg/kg. For comparison, the Indian cobra's (naja naja) subcutaneous LD50 is 0.80 mg/kg, while the Cape cobra's (naja nivea) subcutaneous LD50 is 0.72 mg/kg. This makes the Black desert cobra a more venomous species than both.[67] The venom is strongly neurotoxic and also has mild hemotoxic factors. Envenomation usually causes some combination of local pain, swelling, fever, general weakness, headache, & vomiting. This is not a typically aggressive snake, but it will strike and hiss loudly when provoked. It can strike at a distance of 2/3 of its body length. It does not usually spread a hood nor hold up its body up off the ground like true cobras do. Envenomation by this species should be considered a serious medical emergency. Reports of human fatalities due to envenomation by this species has been reported.[124]
Spitting cobras are another group of cobras that belong the Naja genus. Spitting cobras can be found in both Africa and Asia. These cobras have the ability to eject venom from their fangs when defending themselves against predators. The sprayed venom is harmless to intact skin. However, it can cause permanent blindness if introduced to the eye and left untreated (causing chemosis and corneal swelling). The venom sprays out in distinctive geometric patterns, using muscular contractions upon the venom glands. These muscles squeeze the glands and force the venom out through forward-facing holes at the tips of the fangs.[125] The explanation that a large gust of air is expelled from the lung to propel the venom forward has been proven wrong.[126] When cornered, some species can "spit" their venom a distance as great as 2 m (6.6 ft). While spitting is typically their primary form of defense, all spitting cobras are capable of delivering venom through a bite as well. Most species' venom exhibit significant hemotoxic effects, along with more typical neurotoxic effects of other cobra species. The Philippine cobra (Naja philippinensis) is the third most venomous cobra species in the world based on murine LD50 studies. The subcutaneous LD50 for this species is 0.20 mg/kg and the average venom yield per bite is 90–100 mg.[67] The venom of the Philippine cobra is a potent postsynaptic neurotoxin which affects respiratory function and can cause neurotoxicity and respiratory paralysis, as the neurotoxins interrupt the transmission of nerve signals by binding to the neuromuscular junctions near the muscles. Research has shown its venom is purely a neurotoxin, with no apparent necrotizing components and no cardiotoxins. These snakes are capable of accurately spitting their venom at a target up to 3 metres (9.8 ft) away. Bites from this species produce prominent neurotoxicity and are considered especially dangerous. A study of 39 patients envenomed by the Philippine cobra was conducted in 1988. Neurotoxicity occurred in 38 cases and was the predominant clinical feature. Complete Respiratory failure developed in 19 patients, and was often rapid in onset; in three cases, apnea occurred within just 30 minutes of the bite. There were two deaths, both in patients who were moribund upon arrival at the hospital. Three patients developed necrosis, and 14 individuals with systemic symptoms had no local swelling at all. Both cardiotoxicity and reliable nonspecific signs of envenoming were absent. Bites by the Philippine cobra produce a distinctive clinical picture characterized by severe neurotoxicity of rapid onset and minimal local tissue damage.[127] The Samar cobra (Naja samarensis) is a highly venomous species of spitting cobra that is very closely related to the Philippine cobra, as both are found in that country. The Samar cobra, however, is only found in the south. Although it is a spitting cobra, this species only rarely spits its venom.[128] It is considered to be an extremely aggressive snake that strikes with little provocation. The venom of this species is not well studied, but is known to be an extremely potent postsynaptic neurotoxin that also contains cytotoxic agents.[129] The murine SC LD50 value is 0.46 mg/kg, making it one of the most venomous cobras in the world.[103] Severe envenomation is likely in case of a bite and envenomation rate is high. The untreated mortality rate is not known, but is thought to be high (~60%). Envenomation results in marked local effects such as pain, severe swelling, bruising, blistering, and necrosis. Other effects include headache, nausea, vomiting, abdominal pain, diarrhea, dizziness, collapse or convulsions. There may also be moderate to severe flaccid paralysis and renal damage. Cardiotoxicity is possible, but rare.[128] The Indochinese spitting cobra (Naja siamensis) is a venomous spitting cobra whose venom consists of postsynaptic neurotoxins, metalloproteinases, powerful cardiotoxins, with cytolytic activity, and Phospholipase A2 with a diversity of activities. The LD50 of its venom is 1.07-1.42 mg/gram of mouse body weight.[120] Cranial palsy and respiratory depression are reported to be more common after bites by Naja siamensis than by Naja kaouthia. Indochinese sptting cobras will use their venom for self-defense with little provocation, and as the name implies, are capable of spitting venom when alarmed, often at the face and eyes of the animal or human threatening them. A case report in the literature describes pain and irritation of the eyes, bilateral redness, excessive tear production and whitish discharge, with superficial corneal opacity but normal acuity.[130] The Black-necked spitting cobra (Naja nigricollis) is a species of spitting cobra found mostly in Sub-Saharan Africa. They possess medically significant venom, although the mortality rate for untreated bites on humans is relatively low (~ 5-10%). Like other spitting cobras, this species is known for its ability to project venom at a potential threat. The venom is an irritant to the skin and eyes. If it enters the eyes, symptoms include extreme burning pain, loss of coordination, partial loss of vision and permanent blindness. N. nigricollis is known for its tendency to liberally spit venom with only the slightest provocation. However, this aggressiveness is counterbalanced by it being less prone to bite than other related species.[92][21] The venom of the black-necked spitting cobra is somewhat unique among elapids in that it consists primarily of cytotoxins,[131] but with other components also. It retains the typical elapid neurotoxic properties while combining these with highly potent cytotoxins (necrotic agents)[111] and cardiotoxins.[132] Bite symptoms include severe external hemorrhaging and tissue necrosis around the bite area and difficulty breathing. Although mortality rate in untreated cases is low (~ 5-10%),[133] when death occurs it is usually due to asphyxiation by paralysis of the diaphragm. The LD50 of this species is 2 mg/kg SC and 1.15 mg/kg IV. The average venom yield per bite of this species is 200 to 350 mg ( dry weight ) according to Minton (1974).[92] Another medically important African spitting cobra is the Mozambique spitting cobra (Naja mossambica). This species is considered irritable and highly aggressive. The Mozambique spitting cobra is responsible for a significant amount of bites throughout its range, but most are not fatal. The venom is both neurotoxic and cytotoxic.[134] The Rinkhals (Hemachatus haemachatus) is a not a true cobra in that it does not belong to the genus Naja. However, it is closely related to the true cobras and is considered to be one of the true spitting cobras.[135] The venom of this species is less viscous than that of other African elapids, naturally, as thinner fluid is naturally easier to spit. However, the venom of the rinkhals is produced in copious amounts. Average venom yield is 80-120 mg and the murine LD50 is 1.1-1.6 mg/kg SC with an estimated lethal dose for humans of 50-60 mg. Actual bites from this species are fairly rare, and deaths in modern times are so far unheard of. Local symptoms of swelling and bruising is reported in about 25% of cases. General symptoms of drowsiness, nausea, vomiting, violent abdominal pain and vertigo often occur, as does a mild pyrexial reaction. Neurotoxic symptoms are however rare and have only included diplopia and dyspnoea. Ophthalmia has been reported, but has not caused as severe complications as in some of the spitters in the genus Naja (especially N. nigricollis and N. mossambica).[136]
The Puff adder (Bitis arietans) is responsible for more fatalities than any other African snake. This is due to a combination of factors, including its wide distribution, common occurrence, large size, potent venom that is produced in large amounts, long fangs, their habit of basking by footpaths and sitting quietly when approached.[87][88][89] The venom has cytotoxic effects[137] and is one of the most toxic of any vipers based on LD50 studies.[87] The LD50 values in mice vary: 0.4–2.0 mg/kg IV, 0.9–3.7 mg/kg IP, 4.4–7.7 mg/kg SC.[67] Mallow et al. (2003) gives a LD50 range of 1.0–7.75 mg/kg SC. Venom yield is typically between 100–350 mg, with a maximum of 750 mg.[87] Brown (1973) mentions a venom yield of 180–750 mg.[67] About 100 mg is thought to be enough to kill a healthy adult human male, with death occurring after 25 hours. In humans, bites from this species can produce severe local and systemic symptoms. Based on the degree and type of local effect, bites can be divided into two symptomatic categories: those with little or no surface extravasation, and those with hemorrhages evident as ecchymosis, bleeding and swelling. In both cases there is severe pain and tenderness, but in the latter there is widespread superficial or deep necrosis and compartment syndrome.[138] Serious bites cause limbs to become immovably flexed as a result of significant hemorrhage or coagulation in the affected muscles. Residual induration, however, is rare and usually these areas completely resolve.[87] The fatality rate highly depends on the severity of the bites and some other factors. Deaths can be exceptionally rare and probably occur in less than 10% of all untreated cases (usually in 2–4 days from complications following blood volume deficit and a disseminated intravascular coagulopathy), although some reports show that very severe envenomations have a 52% mortality rate.[54][139] Most fatalities are associated with bad clinical management and neglect.[89][88] The Rhinoceros viper (Bitis nasicornis) is a large species of viper that is similar to the Gaboon viper, but not as venomous, smaller and with a less dangerous bite. They are slow moving, but like other Bitis species, they're capable of striking quickly, forwards or sideways, without coiling first or giving a warning. Holding them by the tail is not safe; as it is somewhat prehensile, they can use it to fling themselves upwards and strike.[87] They have been described as generally placid creatures, not as bad-tempered as the Puff adder. When approached, they often reveal their presence by hissing,[87] said to be the loudest hiss of any African snake—almost a shriek.[89] Relatively little is known about the toxicity and composition of the venom, but it has very minor neurotoxic, as well as hemotoxic venom, as do most other venomous snakes. The hemotoxic venom in rhinoceros vipers is much more dominant. This venom attacks the circulatory system of the snake's victim, destroying tissue and blood vessels. Internal bleeding also occurs. In mice, the intravenous LD50 is 1.1 mg/kg. The venom is supposedly slightly less toxic than those of the Puff adder and the Gaboon viper. The maximum wet venom yield is 200 mg.[88] In only a few detailed reports of human envenomation, massive swelling, which may lead to necrosis, had been described.[88] In 2003, a man in Dayton, Ohio, who was keeping a specimen as a pet, was bitten and subsequently died.[140] At least one antivenom protects specifically against bites from this species: India Antiserum Africa Polyvalent.[141]
The Australian King brown snake or Mulga snake is a the second longest species of venomous snake in Australia. The venom of this snake is relatively weak compared to many other Australian species. The LD50 is 2.38 mg/kg subcutaneous.[142] However, these snakes can deliver large amounts of venom when they bite, compensating for the lower venom potency. Average venom yield is 180 mg and they have a maximum yield of 600 mg.[143][144] The venom of this species contains potent myotoxins and anticoagulants, that can inhibit blood clotting. The neurotoxic components are weak. This snake can cause severe envenomation of humans. They are a moderately common cause of snakebites and uncommonly to rarely cause snakebite deaths in Australia at present. Envenomation can cause anticoagulation coagulopathy, renal damage or renal failure (kidney failure). They do not cause significant neurotoxic paralysis (muscle weakness, respiratory failure), though rarely they may cause ptosis (drooping of the upper eyelids). Bites can also cause myolysis (rhabdomyolysis, muscle damage) which can be very severe and is the major effect of bites.[145] Rate of envenomation is 40-60%, while untreated mortality rate is 30-40%.[146] The Red-bellied black snake (Pseudechis porphyriacus) is a venomous species native to Australia. The venom of the red-bellied black snake consists of myotoxins, coagulants and also has haemolytic and cytotoxic properties. It also contains weak pre-synaptic neurotoxins. The murine LD50 is 2.52 mg/kg SC. Average venom yield per bite is 37 mg and a maximum yield of 97 mg.[143] Bites from red-bellied black snake are rarely life-threatening due to the snake usually choosing to inject little venom toxin, but are still in need of immediate medical attention. Rate of envenomation is 40-60%, but the untreated mortality rate is less than 1%.[147] The Dugite (Pseudonaja affinis) is a highly venomous Australian brown snake species. The venom of this species contains highly potent presynaptic and postsynaptic neurotoxins and procoagulants. The murine LD50 is 0.66 mg/kg SC.[148] The average venom yield per bite is 18 mg (dry weight of milked venom) according to Meier and White (1995). Rate of envenomation is 20-40% and the untreated mortality rate is 10-20 %by cardiac arrest, renal failure, or cerebral hemorrhage. The Western brown snake (Pseudonaja nuchalis) is a highly venomous species of brown snake common throughout northern Australia. Its venom contains powerful neurotoxins, nephrotoxins and a procoagulant, although humans are not usually affected by the neurotoxins.[149] The bite is usually painless and difficult to see due to their small fangs. Human symptoms of a Western Brown snake bite are headache, nausea/vomiting, abdominal pain, severe coagulopathy and sometimes, kidney damage.[150] The LD50 in mice is 0.47 mg/kg and the average venom yield per bite is 18 mg (dry weight of milked venom) according to Meier and White (1995). The western brown snake can cause rapid death in humans by cardiac arrest, renal failure, or cerebral hemorrhage. The envenomation rate is 20-40% and the untreated mortality rate is 10-20%.[151]
Gallery
-
Common krait
-
Common death adder
-
Inland taipan
-
Eastern brown snake
-
Eastern green mamba
-
Saw-scaled viper
-
Jameson's mamba
-
Russell's viper
-
Tiger snake
-
Western green mamba
-
Gaboon viper
-
Indian cobra
Snakes with exaggerated temperaments and venom toxicity
King cobras (Ophiophagus hannah) are not particularly venomous nor are they aggressive or bad tempered. Its venom toxicity is 1.80 mg/kg SC according to Broad et al. (1979).[152] The mean value of subcutaneous LD50 of five wild-caught king cobras in Southeast Asia was determined as 1.93 mg/kg.[153] However, because the king cobra is the largest venomous snake in the world, it can inject very high amounts of venom in a victim. Between 350 to 500 mg (dry weight) of venom can be injected at once (Minton, 1974). In another study by (Broad et al, 1979), the average venom quantity was 421 mg (dry weight of milked venom).[152] The king cobra has a fearsome reputation. When annoyed, it spreads a narrow hood and growls loudly, but scientists claim that their "legendary aggressiveness" is grossly exaggerated.[154] In most of the local encounters with live, wild king cobras, the snakes appear to be of rather placid disposition, and they usually end up being killed or subdued with hardly any hysterics. These support the view that wild king cobras generally have a mild temperament, and despite their frequent occurrence in disturbed and built-up areas, are adept at avoiding humans. Naturalist Michael Wilmer Forbes Tweedie felt that "this notion is based on the general tendency to dramatise all attributes of snakes with little regard for the truth about them. A moment’s reflection shows that this must be so, for the species is not uncommon, even in populated areas, and consciously or unconsciously, people must encounter king cobras quite frequently. If the snake were really habitually aggressive records of its bite would be frequent; as it is they are extremely rare."[155] Mortality rates vary sharply depending on many factors. Most bites involve non-fatal amounts.[156]
The Boomslang (Dispholidus typus) is a rear-fanged venomous colubrid snake. It has a relatively high potency venom ranging from 0.06 - 0.72 mg/kg. This is sufficient to kill mice in 50% of cases, if the venom reaches a vein (LD50).[157] However, boomslangs are timid snakes, and bites generally occur only when people attempt to handle, catch or kill the animal. When confronted and cornered, they inflate their necks and assume their striking "S"-shaped pose. The above data suggest boomslangs are unlikely to be a significant source of human fatalities throughout their distribution range. In 1957, well-known herpetologist Karl Schmidt died after being bitten by a boomslang. D.S. Chapman stated eight serious human envenomations by boomslangs occurred between 1919 and 1962, two of which were fatal. The venom of this species is very slow to act, symptoms may not be manifested until many hours after the bite. While this provides time for procuring the antivenom, it also may lead victims to underestimate the seriousness of the bite. Boomslangs may sometimes fail to inject venom when they bite, so that even after a bite envenomation is not guaranteed.
Rattlesnakes (Crotalus) such as the Eastern diamondback rattlesnake (Crotalus adamanteus) and the Western diamondback rattlesnake (Crotalus atrox) are the leading causes of snakebite incidents in the United States, but they have a low venom potency and are generally not aggressive or combative. The western diamondback rattlesnake has LD50 values of 2.72 mg/kg IV, 20 mg/kg IM and 18.5 mg/kg SC, which is far less toxic than many other snakes and other rattlesnakes. Brown gives the eastern diamondback rattlesnake an average venom yield of 410 mg (dried venom), along with LD50 values of 1.3-2.4 mg/kg IV, 1.7-3.0 mg/kg IP and 14.5–10 mg/kg SC for toxicity, again much less venomous than most snakes.[67] With the exception of the Tiger rattlesnake (Crotalus tigris), the Neotropical rattlesnake (Crotalus durissus) and the Mojave rattlesnake (Crotalus scutulatus), most rattlesnakes are not a serious threat to human life. Bites are easily treatable and mortality rates are very low (≪ 10%).
Society and culture

Snakes were both revered and worshipped and feared by early civilizations. The ancient Egyptians recorded prescribed treatments for snakebites as early as the Thirteenth dynasty in the Brooklyn Papyrus, which includes at least seven venomous species common to the region today, such as the horned vipers.[158] In Judaism, the Nehushtan was a pole with a snake made of copper wrapped around it, similar in appearance to the Rod of Asclepius. The object was considered sacred with the power to heal bites caused by the snakes which had infested the desert, with people merely having to touch it in order to save themselves from imminent death.
Historically, snakebites were seen as a means of execution in some cultures. In medieval Europe, a form of capital punishment was to throw people into snake pits, leaving people to die from multiple venomous bites. A similar form of punishment was common in Southern Han during China's Five Dynasties and Ten Kingdoms period and in India.[159] Snakebites were also used as a form of suicide, most notably by Egyptian queen Cleopatra VII, who reportedly died from the bite of an asp—likely an Egyptian cobra[158][160]—after hearing of Mark Antony's death.
Snakebite as a surreptitious form of murder has been featured in stories such as Sir Arthur Conan Doyle's The Adventure of the Speckled Band, but actual occurrences are virtually unheard of, with only a few documented cases.[159][161][162] It has been suggested that Boris III of Bulgaria, who was allied to Nazi Germany during World War II, may have been killed with snake venom,[159] although there is no definitive evidence. At least one attempted suicide by snakebite has been documented in medical literature involving a puff adder bite to the hand.[163]
References
- ^ a b c d e f g h i j k l Kasturiratne, A.; Wickremasinghe A. R.; de Silva N.; Gunawardena N. K.; et al. (2008). Winkel, Ken (ed.). "The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths". PloS Medicine. 5 (11): e218. doi:10.1371/journal.pmed.0050218. PMC 2577696. PMID 18986210. Retrieved 2009-06-24.
{{cite journal}}: Unknown parameter|author-separator=ignored (help)CS1 maint: unflagged free DOI (link) - ^ "Snake Venom Detection Kit: Detection and Identification of Snake Venom" (PDF). CSL Limited. 2007. Retrieved 2009-11-24.
The physical identification of Australian and Papua New Guinean snakes is notoriously unreliable. There is often marked colour variation between juvenile and adult snakes and wide size, shape and colour variation between snakes of the same species. Reliable snake identification requires expert knowledge of snake anatomy, a snake key and the physical handling of the snake
{{cite journal}}: Cite journal requires|journal=(help) - ^ White, Julian (2006). "Snakebite & Spiderbite: Management Guidelines". Adelaide: Department of Health, Government of South Australia: 1–71. ISBN 0-7308-9551-3. Retrieved 2009-11-24.
The colour of brown snakes is very variable and misleading for identification purposes. They may be brown, red brown, grey, very dark brown and may be plain in color, have speckling, stripes or bands, or have a dark or black head
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b c d e f g h i j k l m n o p Gold, Barry S. (1 April 2002). "Bites of venomous snakes". The New England Journal of Medicine. 347 (5): 347–56. doi:10.1056/NEJMra013477. PMID 12151473.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Kitchens C, Van Mierop L (1987). "Envenomation by the Eastern coral snake (Micrurus fulvius fulvius). A study of 39 victims". JAMA. 258 (12): 1615–18. doi:10.1001/jama.258.12.1615. PMID 3625968.
- ^ a b Chippaux, J.P. (1998). "Snake-bites: appraisal of the global situation" (PDF). Bulletin of the World Health Organization. 76 (5): 515–24. PMC 2305789. PMID 9868843. Retrieved 2009-07-03.
- ^ a b c d e f g h i j Gutiérrez, José María (2007). "Trends in Snakebite Envenomation Therapy: Scientific, Technological and Public Health Considerations". Current Pharmaceutical Design. 13 (28): 2935–50. doi:10.2174/138161207782023784. PMID 17979738.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b MedlinePlus - Snake bites From Tintinalli JE, Kelen GD, Stapcynski JS, eds. Emergency Medicine: A Comprehensive Study Guide. 6th ed. New York, NY: McGraw Hill; 2004. Update Date: 2/27/2008. Updated by: Stephen C. Acosta, MD, Department of Emergency Medicine, Portland VA Medical Center, Portland, OR. Review provided by VeriMed Healthcare Network. Also reviewed by David Zieve, MD, MHA, Medical Director, A.D.A.M., Inc. Retrieved on 19 mars, 2009
- ^ Health-care-clinic.org - Snake Bite First Aid – Snakebite Retrieved on 21 mars, 2009
- ^ Snake bite image example at MDconsult - Patient Education - Wounds, Cuts and Punctures, First Aid for
- ^ Warrell, David A. (1976). "Snake Venom Ophthalmia and Blindness Caused by the Spitting Cobra (Naja Nigricollis) in Nigeria". The American Society of Tropical Medicine and Hygiene. 25 (3): 525–9. PMID 1084700. Retrieved 2009-09-05.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ismail, Mohammad (1993). "The ocular effects of spitting cobras: I. The ringhals cobra (Hemachatus haemachatus) Venom-Induced corneal opacification syndrome". Clinical Toxicology. 31 (1): 31–41. doi:10.3109/15563659309000372. PMID 8433414.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Confronting the Neglected Problem of Snake Bite Envenoming: The Need for a Global Partnership". PLoS Medicine. Retrieved 2012-06-06.
- ^ a b Phillips, Charles M. (2002). "Sea snake envenomation" (PDF). Dermatologic Therapy. 15 (1): 58–61(4). doi:10.1046/j.1529-8019.2002.01504.x. Retrieved 2009-07-24.
- ^ a b Young, Bruce A. (2002). "Do Snakes Meter Venom?". BioScience. 52 (12): 1121–26. doi:10.1641/0006-3568(2002)052[1121:DSMV]2.0.CO;2.
The second major assumption that underlies venom metering is the snake's ability to accurately assess the target
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Young, Bruce A. (2001). "Venom flow in rattlesnakes: mechanics and metering" (PDF). Journal of Experimental Biology. 204 (Pt 24): 4345–4351. PMID 11815658.
With the species and size of target held constant, the duration of venom flow, maximum venom flow rate and total venom volume were all significantly lower in predatory than in defensive strikes
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Deufel, Alexandra (2003). "Feeding in Atractaspis (Serpentes: Atractaspididae): a study in conflicting functional constraints" (PDF). Zoology. 106 (1): 43–61. doi:10.1078/0944-2006-00088. PMID 16351890. Retrieved 2009-08-25.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)[dead link] - ^ a b Jackson, Kate (2003). "The evolution of venom-delivery systems in snakes" (PDF). Zoological Journal of the Linnean Society. 137 (3): 337–354. doi:10.1046/j.1096-3642.2003.00052.x. Retrieved 2009-07-25.
- ^ Fry, Bryan G. (2006). "Early evolution of the venom system in lizards and snakes" (PDF). Nature. 439 (7076): 584–8. doi:10.1038/nature04328. PMID 16292255. Retrieved 2009-09-18.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Russell, Findlay E. (1980). "Snake Venom Poisoning in the United States". Annual Review of Medicine. 31: 247–59. doi:10.1146/annurev.me.31.020180.001335. PMID 6994610.
- ^ a b Spawls, Stephen (1997). The Dangerous Snakes of Africa. Johannesburg: Southern Book Publishers. p. 192. ISBN 1-86812-575-0.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Cite error: The named reference "Spawls" was defined multiple times with different content (see the help page). - ^ a b c d "National geographic- KING COBRA".
They are fiercely aggressive when cornered (line 28–29)
Cite error: The named reference "NG" was defined multiple times with different content (see the help page). - ^ "National geographic-BLACK MAMBA".
when threatened, highly aggressive (line 2–3)
- ^ Kurecki B, Brownlee H (1987). "Venomous snakebites in the United States". Journal of Family Practice. 25 (4): 386–92. PMID 3655676.
- ^ Gold B, Barish R (1992). "Venomous snakebites. Current concepts in diagnosis, treatment, and management". Emerg Med Clin North Am. 10 (2): 249–67. PMID 1559468.
- ^ Suchard, JR (1999). "Envenomations by Rattlesnakes Thought to Be Dead". The New England Journal of Medicine. 340 (24): 1930. doi:10.1056/NEJM199906173402420. PMID 10375322.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Gold BS, Wingert WA (1994). "Snake venom poisoning in the United States: a review of therapeutic practice". South. Med. J. 87 (6): 579–89. doi:10.1097/00007611-199406000-00001. PMID 8202764.
- ^ Pathmeswaran A, Kasturiratne A, Fonseka M, Nandasena S, Lalloo D, de Silva H (2006). "Identifying the biting species in snakebite by clinical features: an epidemiological tool for community surveys". Trans R Soc Trop Med Hyg. 100 (9): 874–8. doi:10.1016/j.trstmh.2005.10.003. PMID 16412486.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chris Thompson. "Treatment of Australian Snake Bites". Australian anaesthetists' website.[dead link]
- ^ "Indian National Snakebite Protocols 2007" (PDF). Indian National Snakebite Protocol Consultation Meeting, 2nd August 2007, Delhi. Retrieved 31 May 2012.
- ^ Currie, Bart J. (2008). "Effectiveness of pressure-immobilization first aid for snakebite requires further study". Emergency Medicine Australasia. 20 (3): 267–270(4). doi:10.1111/j.1742-6723.2008.01093.x. PMID 18549384.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Patrick Walker, J (2013 Jan). "Venomous bites and stings". Current problems in surgery. 50 (1): 9–44. PMID 23244230.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b American College of Medical, Toxicology (2011 Dec). "Pressure immobilization after North American Crotalinae snake envenomation". Journal of medical toxicology : official journal of the American College of Medical Toxicology. 7 (4): 322–3. PMID 22065370.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Wall, C (2012 Sep). "British Military snake-bite guidelines: pressure immobilisation". Journal of the Royal Army Medical Corps. 158 (3): 194–8. PMID 23472565.
{{cite journal}}: Check date values in:|date=(help) - ^ White, Julian (November 1991). "Oxyuranus microlepidotus". Chemical Safety Information from Intergovernmental Organizations. Retrieved 24 July 2009.
Without appropriate antivenom treatment up to 75% of taipan bites will be fatal. Indeed, in the era prior to specific antivenom therapy, virtually no survivors of taipan bite were recorded.
- ^ a b Theakston RD (1997). "An objective approach to antivenom therapy and assessment of first-aid measures in snake bite" (PDF). Ann. Trop. Med. Parasitol. 91 (7): 857–65. doi:10.1080/00034989760626. PMID 9625943.
- ^ Amaral CF, Campolina D, Dias MB, Bueno CM, Rezende NA (1998). "Tourniquet ineffectiveness to reduce the severity of envenoming after Crotalus durissus snake bite in Belo Horizonte, Minas Gerais, Brazil". Toxicon. 36 (5): 805–8. doi:10.1016/S0041-0101(97)00132-3. PMID 9655642.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Watt G, Padre L, Tuazon ML, Theakston RD, Laughlin LW (1988). "Tourniquet application after cobra bite: delay in the onset of neurotoxicity and the dangers of sudden release". Am. J. Trop. Med. Hyg. 38 (3): 618–22. PMID 3275141.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Holstege CP, Singletary EM (2006). "Images in emergency medicine. Skin damage following application of suction device for snakebite". Annals of Emergency Medicine. 48 (1): 105, 113. doi:10.1016/j.annemergmed.2005.12.019. PMID 16781926.
- ^ Alberts M, Shalit M, LoGalbo F (2004). "Suction for venomous snakebite: a study of "mock venom" extraction in a human model". Ann Emerg Med. 43 (2): 181–6. doi:10.1016/S0196-0644(03)00813-8. PMID 14747805.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bush SP, Hegewald KG, Green SM, Cardwell MD, Hayes WK (2000). "Effects of a negative pressure venom extraction device (Extractor) on local tissue injury after artificial rattlesnake envenomation in a porcine model". Wilderness & environmental medicine. 11 (3): 180–8. doi:10.1580/1080-6032(2000)011[0180:EOANPV]2.3.CO;2. PMID 11055564.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Riggs BS, Smilkstein MJ, Kulig KW, et al. Rattlesnake envenomation with massive oropharyngeal edema following incision and suction (Abstract). Presented at the AACT/AAPCC/ABMT/CAPCC Annual Scientific Meeting, Vancouver, Canada, September 27 October 2, 1987.
- ^ Russell F (1987). "Another warning about electric shock for snakebite". Postgrad Med. 82 (5): 32. PMID 3671201.
- ^ Ryan A (1987). "Don't use electric shock for snakebite". Postgrad Med. 82 (2): 42. PMID 3497394.
- ^ Howe N, Meisenheimer J (1988). "Electric shock does not save snakebitten rats". Ann Emerg Med. 17 (3): 254–6. doi:10.1016/S0196-0644(88)80118-5. PMID 3257850.
- ^ Johnson E, Kardong K, Mackessy S (1987). "Electric shocks are ineffective in treatment of lethal effects of rattlesnake envenomation in mice". Toxicon. 25 (12): 1347–9. doi:10.1016/0041-0101(87)90013-4. PMID 3438923.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Russell, F. E. (1990). "When a snake strikes". Emerg Med. 22 (12): 33–4, 37–40, 43.
- ^ a b Mackessy, Stephen P. (2002). "Biochemistry and pharmacology of colubrid snake venoms" (PDF). Journal of Toxicology: Toxin Reviews. 21 (1–2): 43–83. doi:10.1081/TXR-120004741. Retrieved 2009-09-26.
Estimates of the number of venomous colubrids approach 700 species. Most may not produce a venom capable of causing serious damage to humans, but at least five species (Dispholidus typus, Thelotornis capensis, Rhabdophis tigrinus, Philodryas olfersii and Tachymenis peruviana) have caused human fatalities
- ^ a b Wingert W, Chan L (1 January 1988). "Rattlesnake Bites in Southern California and Rationale for Recommended Treatment". West J Med. 148 (1): 37–44. PMC 1026007. PMID 3277335.
- ^ Gutiérrez, José María (6 June 2006). "Confronting the Neglected Problem of Snake Bite Envenoming: The Need for a Global Partnership". PLOS Medicine. 3 (6): e150. doi:10.1371/journal.pmed.0030150. PMC 1472552. PMID 16729843. Retrieved 2009-06-30.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Parrish H (1966). "Incidence of treated snakebites in the United States". Public Health Rep. 81 (3): 269–76. doi:10.2307/4592691. PMC 1919692. PMID 4956000.
- ^ a b Zug, George R. (1996). Snakes in Question: The Smithsonian Answer Book. Washington D.C., USA: Smithsonian Institution Scholarly Press. ISBN 1-56098-648-4.
- ^ a b White, Nancy (2009). Black Mambas: Sudden Death!. Bearport Publishing. ISBN 1597167665.
- ^ a b c d e Davidson, Terence. "IMMEDIATE FIRST AID". University of California, San Diego. Cite error: The named reference "Davidson" was defined multiple times with different content (see the help page).
- ^ Crisp, NG (31). "Black mamba envenomation". South African Medical Journal. 68 (5): 293–4. PMID 4035489.
{{cite journal}}:|access-date=requires|url=(help); Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|month=ignored (help) - ^ Hilligan, R (1). "Black mamba bites. A report of 2 cases". South African Medical Journal. 72 (3): 220–1. PMID 3603321. Retrieved 20 October 2013.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|month=ignored (help) - ^ a b "IMMEDIATE FIRST AID for bites by Australian taipan or common taipan".
- ^ "University of Adelaide Clinical Toxinology Resources".
- ^ Pitman, Charles R.S. (1974). A Guide to the Snakes of Uganda. United Kingdom: Wheldon & Wesley. p. 290. ISBN 0-85486-020-7.
- ^ "Coastal Taipan". Queensland Museum. Queensland Government. Retrieved 21 October 2013.
- ^ a b c d Whitaker, Romulus. "Publicity Notes One Million Snake Bite" (PDF). IconFilms. Retrieved 21 October 2013.
- ^ "University of Adelaide Clinical Toxinology Resources".
Mortality rate:70-80%
- ^ Tan, Nget Hong. "Toxins from Venoms of Poisonous Snake Indigenous to Malaysia: A Review". Department of Molecular Medicine, Faculty of Medicine. University of Malaya. Retrieved 21 October 2013.
- ^ "University of Adelaide Clinical Toxinology Resources".
Mortality rate:70%
- ^ "Clinical Toxinology-Bungarus multicinctus".
- ^ a b c Snake of medical importance. Singapore: Venom and toxins research group. ISBN 9971-62-217-3.
- ^ a b c d e f g h i j k l m n o p q r s t u Brown JH. 1973. Toxicology and Pharmacology of Venoms from Poisonous Snakes. Springfield, Illinois: Charles C. Thomas. 184 pp. LCCCN 73-229. ISBN 0-398-02808-7. Cite error: The named reference "Bro73" was defined multiple times with different content (see the help page).
- ^ a b "LD50 menu".
- ^ Chi, Wen Juan (29). "Venomous Snake Bites in Taiwan" (PDF). Journal of Critical Care and Emergency Medicine. 23 (4): 98. Retrieved 22 October 2013.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|month=ignored (help) - ^ a b "LD50 Scores for various snakes". Retrieved 22 October 2013.
- ^ a b c "University of Adelaide Clinical Toxinology Resources".
Mortality rate:80%
- ^ "University of Adelaide Clinical Toxinology Resources".
- ^ "Australia's 10 most dangerous snakes". Australian Geographic. Australian Geographic. Retrieved 20 October 2013.
- ^ "University of Adelaide Clinical Toxinology Resources".
Mortality rate:50-60%
- ^ a b Fastest striking snake
- ^ "LD50 of venomous snakes - Ultimate species list". Snake Database. Retrieved 21 October 2013.
- ^ "Common death adder Venom Yield". Retrieved 21 October 2013.
- ^ "Australia's 10 most dangerous snakes". Australian Geographic. Australian Geographic. Retrieved 20 October 2013.
- ^ University of Adelaide Clinical Toxinology Resource
- ^ "Australian Tiger Snakes". Clinical Toxinology Resources. University of Adelaide. Retrieved 22 October 2013.
- ^ McDiarmid RW, Campbell JA, Touré T. 1999. Snake Species of the World: A Taxonomic and Geographic Reference, vol. 1. Herpetologists' League. 511 pp. ISBN 1-893777-00-6 (series). ISBN 1-893777-01-4 (volume).
- ^ "Saw-scaled viper". Encyclopedia Britannica. Encyclopedia Britannica. Retrieved 20 October 2013.
- ^ Mackessy 2010, p. 456
- ^ "University of Adelaide Clinical Toxinology Resources".
- ^ Daniels,J. C. (2002) The Book of Indian Reptiles and Amphibians, BNHS & Oxford University Press, Mumbai, pp 151-153. ISBN 0-19-566099-4
- ^ a b Ali G, Kak M, Kumar M, Bali SK, Tak SI, Hassan G, Wadhwa MB. 2004. Acute renal failure following echis carinatus (saw–scaled viper) envenomation. Indian Journal of Nephrology 14:177-181. PDF at Indian Medlars Centre. Accessed 12 September 2006.
- ^ a b c d e f g h i j k l Mallow D, Ludwig D, Nilson G. 2003. True Vipers: Natural History and Toxinology of Old World Vipers. Malabar, Florida: Krieger Publishing Company. 359 pp. ISBN 0-89464-877-2. Cite error: The named reference "Mal03" was defined multiple times with different content (see the help page).
- ^ a b c d e f g Spawls S, Branch B. 1995. The Dangerous Snakes of Africa. Ralph Curtis Books. Dubai: Oriental Press. 192 pp. ISBN 0-88359-029-8.
- ^ a b c d e f Spawls S, Howell K, Drewes R, Ashe J. 2004. A Field Guide To The Reptiles Of East Africa. London: A & C Black Publishers Ltd. 543 pp. ISBN 0-7136-6817-2.
- ^ a b c Engelmann, Wolf-Eberhard (1981). Snakes: Biology, Behavior, and Relationship to Man. Leipzig; English version NY, USA: Leipzig Publishing; English version published by Exeter Books (1982). p. 51. ISBN 0-89673-110-3. Cite error: The named reference "Engelmann" was defined multiple times with different content (see the help page).
- ^ Brown, John H. (1973). Toxicology and Pharmacology of Venoms from Poisonous Snakes. Springfield, IL USA: Charles C. Thomas. p. 81. ISBN 0-398-02808-7.
- ^ a b c d Clinical Toxinology Resource (Dendroaspis jamesoni) Cite error: The named reference "WCH" was defined multiple times with different content (see the help page).
- ^ Whitaker Z. 1990. Snakeman. Penguin Books Ltd. 192 pp. ISBN 0-14-014308-4.
- ^ Warrell, David A. "Clinical Features of Snakebite". Encyclopedia of Occupational Health and Safety. Encyclopedia of Occupational Health and Safety. Retrieved 21 October 2013.
- ^ a b "Naja naja". University of Adelaide.
- ^ Whitaker, Captain, Romulus, Ashok (2004). Snakes of India, The Field Guide. India: Draco Books. p. 372. ISBN 81-901873-0-9.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ World Health Organization. "Zoonotic disease control: baseline epidemiological study on snake-bite treatment and management". Weekly Epidemiological Record (WER). 62 (42): 319–320. ISSN 0049-8114.
- ^ a b c Campbell; Lamar, Jonathan; William (2004). The Venomous Reptiles of the Western Hemisphere. Ithaca and London: Comstock Publishing Associates. p. 870. ISBN 0-8014-4141-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Sierra. "Captive care of B.asper". A collection of captive care notes. www.venomousreptiles.org. Retrieved 6 November 2006.
- ^ a b Warrell DA. 2004. Snakebites in Central and South America: Epidemiology, Clinical Features, and Clinical Management. In Campbell JA, Lamar WW. 2004. The Venomous Reptiles of the Western Hemisphere. Comstock Publishing Associates, Ithaca and London. 870 pp. 1500 plates. ISBN 0-8014-4141-2.
- ^ "Clodomiro Picado Research Institute".
- ^ a b c d Weinstein, Scott A. (30). "Lethal toxins and cross-neutralization of venoms from the African water cobras, Boulengerina annulata annulata and Boulengerina christyi". Toxicon. 29 (11): 1315–1327. PMID 1814007.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b Wüster, W (1). "Asiatic cobras: Systemics and snakebite". Experientia. 47 (2): 205–209. doi:10.1007/BF01945429.
{{cite journal}}:|access-date=requires|url=(help); Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Yap, MKK (2011). "Biochemical and toxinological characterization of Naja sumatrana (Equatorial spitting cobra) venom". The Journal of Venomous Animals and Toxins including Tropical Diseases. 17 (4): 451–459. doi:10.1590/S1678-91992011000400012.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Snakes of medical importance. Singapore: Venom and toxic research group. p. 253. ISBN 9971-62-217-3.
- ^ a b Warrell, David A. "Guidelines for the Prevention and Clinical Management of Snakebite in Africa". World Health Organization. Retrieved 23 October 2013.
- ^ "IMMEDIATE FIRST AID for bites by Forest Cobra (Naja melanoleuca)". Retrieved 22 October 2013.
- ^ Wang, AH (10). "Crystallographic studies of snake venom proteins from Taiwan cobra (Naja nana atra). Cardiotoxin-analogue III and phospholipase A2" (PDF). The Journal of Biological Chemistry. 256 (17): 9279–9282. PMID 7263715. Retrieved 21 October 2013.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b Snake of medical importance. Singapore: Venom and toxins research group. ISBN 9971-62-217-3.
- ^ "University of Adelaide Clinical Toxinology Resources".
- ^ a b Sharonov, George V.; Feofanov, Alexei V.; Astapova, Maria V.; Rodionov, Dmitriy I.; Utkin, Yuriy N.; Arseniev, Alexander S. (2005). "Cancer cell injury by cytotoxins from cobra venom is mediated through lysosomal damage". Biochemical Journal. 390 (Pt 1): 11–8. doi:10.1042/BJ20041892. PMC 1184559. PMID 15847607. Cite error: The named reference "cytotoxins" was defined multiple times with different content (see the help page).
- ^ Dementieva, Daria V.; Bocharov, Eduard V.; Arseniev, Alexander. S. (1999). "Two forms of cytotoxin II (cardiotoxin) from Naja naja oxiana in aqueous solution . Spatial structures with tightly bound water molecules". European Journal of Biochemistry. 263 (1): 152–62. doi:10.1046/j.1432-1327.1999.00478.x. PMID 10429199.
- ^ Gopalkrishnakone, Chou, P., LM (1990). Snakes of Medical Importance (Asia-Pacific Region). Singapore: National University of Singapore. ISBN 9971-62-217-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Morgan, Haagner, Dave, Gerald. "Husbandry and Propagation of the Cape cobra (Naja nivea) at the Manyeleti Reptile Centre (pg 1)". The Journal of Herpetological Association of Africa. Retrieved 23 October 2013.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ "IMMEDIATE FIRST AID For bites by Cape Cobra (Naja nivea)". University of California, San Diego. University of California, San Diego. Retrieved 22 October 2013.
- ^ Wei, J.-F., Lü, Q.-M., Jin, Y., Li, D.-S., Xiong, Y.-L., Wang, W.-Y. (2003). [http://www.abbs.info/fulltxt/eng/35080683.htm α-Neurotoxins of Naja atra and Naja kaouthia Snakes in Different Regions]. Acta Biochimica et Biophysica Sinica 35 (8): 683–688.
- ^ Ogay, A. (2005). "Weak neurotoxin from Naja kaouthia cobra venom affects haemodynamic regulation by acting on acetylcholine receptors". Toxicon. 45 (1): 93–99. doi:10.1016/j.toxicon.2004.09.014.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Mahanta, M. (2001). "Neutralisation of lethality, myotoxicity and toxic enzymes of Naja kaouthia venom by Mimosa pudica root extracts". Journal of Ethnopharmacology. 75 (1): 55–60. doi:10.1016/S03788741(00)003731.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Fletcher, J. E. (1991). "Effects of a cardiotoxin from Naja naja kaouthia venom on skeletal muscle: Involvement of calcium-induced calcium release, sodium ion currents and phospholipases A2 and C". Toxicon. 29 (12): 1489–1500. doi:10.1016/0041-0101(91)90005-C.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Chanhome, L., Cox, M. J., Vasaruchaponga, T., Chaiyabutra, N. Sitprija, V. (2011). Characterization of venomous snakes of Thailand. Asian Biomedicine 5 (3): 311–328.
- ^ Pratanaphon, R. (1997). "Production of highly potent horse antivenom against the Thai cobra (Naja kaouthia)". Vaccine. 15 (14): 1523–1528. doi:10.1016/S0264-410X(97)00098-4.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Joubert, FJ (1978). "Naja haje haje (Egyptian cobra) venom. Some properties and the complete primary structure of three toxins (CM-2, CM-11 and CM-12)". European Journal of Biochemistry. 90 (2): 359–367. PMID 710433.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ "Venomous Animals - Boulengerina annulata and Boulengerina christyi". Armed Forces Pest Management Board. United States Army. Retrieved 24 October 2013.
- ^ "Venomous Animals - Walterinnesia aegyptia". Armed Forces Pest Management Board. United States Army. Retrieved 24 October 2013.
- ^ Young, B. A.; Dunlap, K.; Koenig, K.; Singer, M. (2004). "The buccal buckle: The functional morphology of venom spitting in cobras". Journal of Experimental Biology. 207 (20): 3483–3494. doi:10.1242/jeb.01170. PMID 15339944.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Rasmussen, Sara; Young, B.; Krimm, Heather (1995). "On the 'spitting' behaviour in cobras (Serpentes: Elapidae)". Journal of Zoology. 237 (1): 27–35. doi:10.1111/j.1469-7998.1995.tb02743.x.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Watt, G. (1988). "Bites by the Philippine cobra (Naja naja philippinensis): prominent neurotoxicity with minimal local signs". The American Journal of Tropical Medicine and Hygiene. 39 (3): 306–11. PMID 3177741. Retrieved 1 December 2011.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b "Naja samarensis". University of Adelaide.
- ^ Dart, Richard C (2003). Medical Toxicology. USA: Lippincott Williams & Wilkins; 3 edition. p. 1569. ISBN 0-7817-2845-2.
- ^ Williams, Jensen, O'Shea, David J., Simon D., Mark. "Snake Management in Cambodia" (PDF). Retrieved 23 October 2013.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Marais, Johan (2004). A Complete Guide to the Snakes of Southern Africa. Cape Town, South Africa: Struik Nature. ISBN 1-86872-932-X.
- ^ Fryklund, Linda (1975). "Complete covalent structure of a cardiotoxin from the venom of Naja nigricollis (African black-necked spitting cobra)". Biochemistry. 14 (13): 2865–2871. doi:10.1021/bi00684a012. PMID 1148181.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Warrell, David A. "Snake bite" (PDF). Seminar. Lancet 2010 (volume 375, issue 1).
- ^ Tilbury, CR. "Observations on the bite of the Mozambique spitting cobra" (PDF). Retrieved 23 October 2013.
- ^ S. Hunter (2000). "Venomous Reptiles".
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^ "The Natural History and Captive Care of the Rinkhals spitting cobra". Retrieved 23 October 2013.
- ^ Widgerow AD, Ritz M, Song C. 1994. Load cycling closure of fasciotomies following puff adder bite. European Journal of Plastic Surgery 17: 40-42. Summary at Springerlink. Accessed 23 October 2013.
- ^ Rainer PP, Kaufmann P, Smolle-Juettner FM, Krejs GJ (2010). "Case report: Hyperbaric oxygen in the treatment of puff adder (Bitis arietans) bite". Undersea & Hyperbaric Medicine : Journal of the Undersea and Hyperbaric Medical Society, Inc. 37 (6): 395–398. PMID 21226389.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ U.S. Navy. 1991. Venomous Snakes of the World. US Govt. New York: Dover Publications Inc. 203 pp. ISBN 0-486-26629-X.
- ^ Firefighter Dies After Bite From Pet Snake at channelcincinnati.com. Accessed 24 October 2013.
- ^ Miami-Dade Fire Rescue Venom Response Unit at VenomousReptiles.org. Accessed 24 October 2013.
- ^ The Australian venom research unit (August 25, 2007). "Which snakes are the most venomous?". University of Melbourne. Retrieved October 24, 2013.
- ^ a b Shea, Glenn (1999). "The distribution and identification of dangerously venomous Australian terrestrial snakes". Australian Veterinary Journal. 77 (12): 791–798.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|month=ignored (help) - ^ Sutherland, SK (1983). Australian Animal Toxins. OUP Australia and New Zealand. ISBN 019554367X.
- ^ "Australian Mulga Snakes". Clinical Toxinology Resource. University of Adelaide. Retrieved 24 October 2013.
- ^ "University of Adelaide Clinical Toxinology Resources".
Mortality rate:30-40%
- ^ "University of Adelaide Clinical Toxinology Resources".
Mortality rate:<1%
- ^ Cheng, David. "Brown Snake Envenomation". Retrieved 24 October 2013.
- ^ Venom Supplies Pty Ltd. "Brown Snakes".
- ^ Toxinology Department, Women's & Children's Hospital, Adelaide, Australia. "CSL Antivenom Handbook - Brown Snake Antivenom".
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ "University of Adelaide Clinical Toxinology Resources".
Mortality rate:10-20%
- ^ a b University of Adelaide Clinical Toxinology Resource
- ^ Handbook of clinical toxicology of animal venoms and poisons. Vol. 236. USA: CRC Press. 1995. ISBN 0-8493-4489-1.
- ^ Greene, HW (1997). Snakes: The Evolution of Mystery in Nature. California, USA: University of California Press. ISBN 0520224876.
- ^ Tweedie, MWF (1983). The Snakes of Malaya. Singapore: Singapore National Printers Ltd. ISBN B0007B41IO.
{{cite book}}: Check|isbn=value: invalid character (help) - ^ Mathew, Gera, JL, T. "http://www.priory.com/med/ophitoxaemia.htm". MEDICINE ON-LINE. Retrieved 20 October 2013.
{{cite web}}: External link in|title= - ^ Stephen P. Mackessy: Biochemistry and Pharmacology of Colubrid Snake Venoms. J. Toxicol.—Toxin Reviews 21 (1&2), 2002: pp. 43–83 online PDF
- ^ a b Schneemann, M. (2004). "Life-threatening envenoming by the Saharan horned viper (Cerastes cerastes) causing micro-angiopathic haemolysis, coagulopathy and acute renal failure: clinical cases and review" (PDF). QJM: an International Journal of Medicine. 97 (11): 717–27. doi:10.1093/qjmed/hch118. PMID 15496528. Retrieved 2009-09-04.
This echoed the opinion of the Egyptian physicians who wrote the earliest known account of the treatment of snake bite, the Brooklyn Museum Papyri, dating perhaps from 2200 BC. They regarded bites by horned vipers 'fy' as non-lethal, as the victims could be saved.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Anil, Aggrawal (2004). "Homicide with snakes: A distinct possibility and its medicolegal ramifications". Anil Aggrawal's Internet Journal of Forensic Medicine and Toxicology. 4 (2).
- ^ Crawford, Amy (April 1, 2007). "Who Was Cleopatra? Mythology, propaganda, Liz Taylor and the real Queen of the Nile". Smithsonian.com. Retrieved 4 September 2009.
- ^ Warrell, D.A. (2009). "Commissioned article: management of exotic snakebites". QJM: an International Journal of Medicine. 102 (9): 593–601. doi:10.1093/qjmed/hcp075. PMID 19535618.
- ^ Straight, Richard C. (1994). "Human fatalities caused by venomous animals in Utah, 1900–90". Great Basin Naturalist. 53 (4): 390–4. Retrieved 2009-09-04.
A third unusual death was a tragic fatality (1987), recorded as a homicide, which resulted when a large rattlesnake (G. v. lutosus) bit a 22-month-old girl after the snake had been placed around her neck (Washington County). The child died in approximately 5 h.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Strubel, T. (2008). "Suizidversuch durch Schlangenbiss: Kasuistik und Literaturübersicht". Der Nervenarzt (in German). 79 (5): 604–6. doi:10.1007/s00115-008-2431-4. PMID 18365165.
Ein etwa 20-jähriger Arbeiter wurde nach dem Biss seiner Puffotter (Bitis arietans) in die Hand auf die toxikologische Intensivstation aufgenommen. Zunächst berichtet der Patient, dass es beim „Melken" der Giftschlange zu dem Biss gekommen sei, erst im weiteren Verlauf räumt er einen Suizidversuch ein. Als Gründe werden Einsamkeit angeführt sowie unerträgliche Schmerzen im Penis.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|trans_title=ignored (|trans-title=suggested) (help)
- Bibliography
- Greene, Harry W. (1997). Snakes: The Evolution of Mystery in Nature. Berkeley, CA: University of California Press. ISBN 978-0-520-20014-2.
{{cite book}}: Invalid|ref=harv(help) - Stephen P., Mackessy, ed. (2010). Handbook of Venoms and Toxins of Reptiles (2nd ed.). Boca Raton, FL: CRC Press. ISBN 978-0-8493-9165-1.
{{cite book}}: Invalid|ref=harv(help) - Valenta, Jiri (2010). Venomous Snakes: Envenoming, Therapy (2nd ed.). Hauppauge, NY: Nova Science Publishers. ISBN 978-1-60876-618-5.
{{cite book}}: Invalid|ref=harv(help)
Further reading
- Campbell, Jonathan A.; William W. Lamar (2004). The Venomous Reptiles of the Western Hemisphere. Ithaca, NY: Cornell University Press. ISBN 978-0-8014-4141-7
- Spawls, Stephen; Bill Branch (1995). The Dangerous Snakes of Africa: Natural History, Species Directory, Venoms and Snakebite. Sanibel Island, FL: Ralph Curtis Publishing. ISBN 978-0-88359-029-4
- Sullivan JB, Wingert WA, Norris Jr RL. (1995). North American Venomous Reptile Bites. Wilderness Medicine: Management of Wilderness and Environmental Emergencies. 3: 680–709.
- Thorpe, Roger S.; Wolfgang Wüster, Anita Malhotra (1996). Venomous Snakes: Ecology, Evolution, and Snakebite'. Oxford, England: Oxford University Press. ISBN 978-0-19-854986-4