Rylene dye

A rylene dye is a dye based on the rylene framework of naphthalene units linked in peri-positions. In homologues additional naphthalene units are added, forming compounds — or poly(peri-naphthalene)s — such as perylene, terrylene and quarterrylene.
Perylene dyes
[edit]Perylene dyes are useful for their intense visible light absorption, high stability, electron accepting ability, and unity quantum yields.[1] Due to these properties, they are actively researched in academia for optoelectronic and photovoltaic devices, thermographic processes, energy-transfer cascades, light-emitting diodes, and near-infrared-absorbing systems.[2]
Molecular design features
[edit]Introduction of carboxylic acid and sulfonic acid groups increases the solubility of rylene dyes in water. The imide structure allows for two different substituents, so a single reactive group can be attached to this position.[2] The HOMO/LUMO levels of perylene diimide derivatives can easily be tuned via substitution at the bay position. A bathochromic shift of about 100 nm is recorded per additional naphthalene unit.
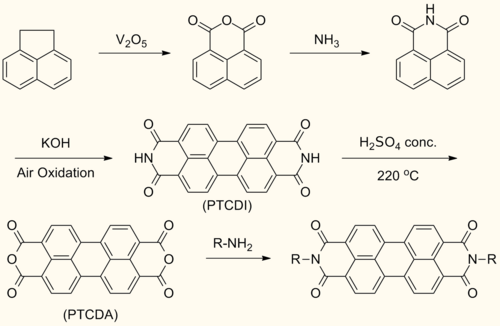
Synthesis
[edit]Perylenediimide (PDI) are synthesized by treating perylenetetracarboxylic dianhydride (PTCDA) with amines at high temperatures.[1]
This reaction forms symmetrically N,N’- substituted PDIs. Solubilizing groups are often attached in this fashion. Although the solubility of the dianhydride is low, the solubility of some mono- and diimide derivatives are greatly improved.
Pigments
[edit]Perylene diimide derivatives were developed as industrial dyes due to their excellent chemical, photo, thermal, and weather stability. Nowadays, perylene dyes are used predominantly in textile applications and as high-grade industrial paint.[1][3][4]

Several perylene pigments have been developed for artist's paints :
- Pigment red 149, a middle red, between light and dark cadmium red. (chemical structure: replace HN group in PV29 with 3,5-(CH3)2C6H3).
- Pigment Red 179, which is close to alizarin crimson (chemical structure: replace HN group in PV29 with CH3N).
- Perylene black 31 (chemical structure: replace HN group in PV29 with C6H5CH2CH2N)
- Rylene dyes have been less popular as fluorescent tags due to their low solubility in aqueous solutions.
Molecular electronics
[edit]Perylenediimide derivatives have been incorporated as n-channel field-effect transistors due to their strong electron affinities. In particular, OFETs using highly packed perylene diimide derivatives with electron withdrawing groups such were found to have good air stability.[5] Close molecular packing is important in retaining air stability. Electron transfer, on the other hand, was strongly influenced by the π-π stacking between perylene diimide units.[6]
Perylene diimide derivatives are suitable as acceptor materials due to their high electron affinity and high electron mobilities.[1][7]
Additional reading
[edit]References
[edit]- ^ a b c d Huang, C., Barlow,S., Marder, S. (2011), Perylene-3,4,9,10-tetracarboxylic Acid Diimides: Synthesis, Physical Properties, and Use in Organic Electronics. Journal of Organic Chemistry, 76, 2386–2407. doi:10.1021/jo2001963
- ^ a b Weil, T., Vosch, T., Hofkens, J., Peneva, K. and Müllen, K. (2010), The Rylene Colorant Family—Tailored Nanoemitters for Photonics Research and Applications. Angewandte Chemie International Edition, 49: 9068–9093. doi:10.1002/anie.200902532
- ^ K. Hunger. W. Herbst "Pigments, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.a20_371
- ^ Greene, M. "Perylene Pigments" in High Performance Pigments, 2009, Wiley-VCH, Weinheim. doi:10.1002/9783527626915.ch16 pp. 261-274.
- ^ Jones, B. A.; Ahrens, M. J.; Yoon, M.-H.; Facchetti, A.; Marks, T. J.; Wasielewski, M. R. (2004) Electron transport: High-mobility air-stable n-type semiconductors with processing versatility: dicyanoperylene-3,4:9,10-bis(dicarboximides). Angew. Chem. Int. Ed., 43, 6363– 6366
- ^ Mei, J., Diao, Y., Appleton, A. L., Fang, L., Bao, Z. (2013), Integrated Materials Design of Organic Semiconductors for Field-Effect Transistors. J. Am. Chem. Soc. 135, 6724
- ^ Usta, H., Facchetti, A., Marks, T. J. (2011) n-Channel Semiconductor Materials Design for Organic Complementary Circuits. Acc. Chem. Res. 44, 501−510