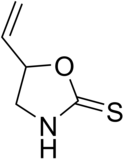
Goitrin
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
5-Vinyl-1,3-oxazolidine-2-thione | |
| Other names
Goitrin
| |
| Identifiers | |
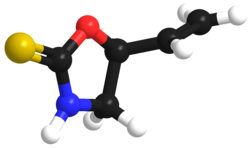
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.032.845 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H7NOS | |
| Molar mass | 129.18 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Goitrin is an organosulfur compound classified as a derivative of oxazolidine and as a cyclic thiocarbamate. It reduces the production of thyroid hormones such as thyroxine.[1] It is found in cruciferous vegetables such as cabbage, brussels sprouts and rapeseed oil,[2] and is formed by the hydrolysis of a glucosinolate: progoitrin or 2-hydroxy-3-butenyl glucosinolate. The unstable isothiocyanate (2-hydroxy-3-butenyl isothiocyanate) derived from the latter glucosinolate spontaneously cyclizes to goitrin, because the hydroxy group is situated in proximity to the isothiocyanate group (allowing a five-membered ring to be formed). Hence, the oxygen in the molecule stems from the hydroxy group of the original unstable isothiocyanate. Plants containing this specific glucosinolate (or glucosinolates such as glucobrassicin and sinalbin which liberate thiocyanate ion) have goitrogenic potential due to the goitrin and thiocyanate they contain. However, they do not seem to alter thyroid function in humans at realistic amounts in the diet.[3]
See also[edit]
References[edit]
- ^ McMillan M, Spinks EA, Fenwick GR (January 1986). "Preliminary Observations on the Effect of Dietary Brussels Sprouts on Thyroid Function". Hum Toxicol. 5 (1): 15–19. doi:10.1177/096032718600500104. PMID 2419242.
- ^ Lüthy J, Carden B, Friederich U, Bachmann M (May 1984). "Goitrin — a nitrosatable constituent of plant foodstuffs". Experientia. 40 (5): 452–453. doi:10.1007/BF01952381. PMID 6723906.
- ^ Verhoeven DT, Verhagen H, Goldbohm RA, van den Brandt PA, van Poppel G (February 1997). "A review of mechanisms underlying anticarcinogenicity by brassica vegetables". Chem. Biol. Interact. 103 (2): 79–129. doi:10.1016/S0009-2797(96)03745-3. PMID 9055870.
