Cooking oil
| Plant oils |
|---|
 |
| Types |
| Uses |
| Components |
Cooking oil (also known as edible oil) is a plant or animal liquid fat used in frying, baking, and other types of cooking. Oil allows higher cooking temperatures than water, making cooking faster and more flavorful, while likewise distributing heat, reducing burning and uneven cooking. It sometimes imparts its own flavor. Cooking oil is also used in food preparation and flavoring not involving heat, such as salad dressings and bread dips.
Cooking oil is typically a liquid at room temperature, although some oils that contain saturated fat, such as coconut oil, palm oil and palm kernel oil are solid.[1]
There are a wide variety of cooking oils from plant sources such as olive oil, palm oil, soybean oil, canola oil (rapeseed oil), corn oil, peanut oil, sesame oil, sunflower oil and other vegetable oils, as well as animal-based oils like butter and lard.
Oil can be flavored with aromatic foodstuffs such as herbs, chilies or garlic. Cooking spray is an aerosol of cooking oil.
Health and nutrition
[edit]While consumption of small amounts of saturated fats is common in diets,[2] meta-analyses found a significant correlation between high consumption of saturated fats and blood LDL concentration,[3] a risk factor for cardiovascular diseases.[4] Other meta-analyses based on cohort studies and on controlled, randomized trials found a positive,[5] or neutral,[6] effect from consuming polyunsaturated fats instead of saturated fats (a 10% lower risk for 5% replacement).[6]
Mayo Clinic has highlighted certain oils that are high in saturated fats, including coconut, palm oil and palm kernel oil. Those having lower amounts of saturated fats and higher levels of unsaturated (preferably omega-3) fats like olive oil, peanut oil, canola oil, soy and cottonseed oils are generally healthier.[7] The US National Heart, Lung and Blood Institute[8] urged saturated fats be replaced with polyunsaturated and monounsaturated fats, listing olive and canola oils as sources of healthier monounsaturated oils while soybean and sunflower oils as good sources of polyunsaturated fats. One study showed that consumption of non-hydrogenated unsaturated oils like soybean and sunflower is preferable to the consumption of palm oil for lowering the risk of heart disease.[9]
Cashew oil and other nut-based oils do not present a danger to persons with a nut allergy, because oils are primarily lipids, and allergic reactions are due to surface proteins on the nut.[10]
The seeds of most cultivated plants contain higher levels of omega-6 fatty acids than omega-3, with some notable exceptions. Growth at colder temperatures tends to result in higher levels of omega-3 fatty acids in seed oils.[11]
Trans fats
[edit]Unlike other dietary fats, trans fats are not essential, and they do not promote good health.[12] The consumption of trans fats increases one's risk of coronary heart disease[13] by raising levels of LDL cholesterol and lowering levels of HDL cholesterol.[14] Trans fats from partially hydrogenated oils are more harmful than naturally occurring oils.[15]
Several large studies[16][17][18][19] indicate a link between the consumption of high amounts of trans fat and coronary heart disease, and possibly some other diseases. The United States Food and Drug Administration (FDA), the National Heart, Lung and Blood Institute and the American Heart Association (AHA) all have recommended limiting the intake of trans fats. In the US, trans fats are no longer "generally recognized as safe", and cannot be added to foods, including cooking oils, without special permission.[20]
Cooking with oil
[edit]


Heating, as well as heating vessels rapidly change characteristics of cooking oil.[21] Oils that are healthy at room temperature can become unhealthy when heated above certain temperatures, especially when heating repeatedly. The toxic risk is linked to oxidation of fatty acids and fatty acids with higher levels of unsaturation are oxidized more rapidly during heating in air.[22] So, when choosing a cooking oil, it is important to match the oil's heat tolerance with the temperature which will be used.[23] and to change frying oil a few times per week.[22] Deep-fat frying temperatures are commonly in the range of 170–190 °C (338–374 °F), less commonly, lower temperatures ≥ 130 °C (266 °F) are used.[24]
Palm oil contains more saturated fats than canola oil, corn oil, linseed oil, soybean oil, safflower oil, and sunflower oil. Therefore, palm oil can withstand deep frying at higher temperatures and is resistant to oxidation compared to high-polyunsaturated vegetable oils.[25] Since the 1900s, palm oil has been increasingly added into food by the global commercial food industry because it remains stable in deep frying, or in baking at very high temperatures,[26][27] and for its high levels of natural antioxidants, though the refined palm oil used in industrial food has lost most of its carotenoid content (and its orange-red color).[28]
The following oils are suitable for high-temperature frying due to their high smoke point:
- Avocado oil
- Mustard oil
- Palm oil
- Peanut oil (marketed as "groundnut oil" in the UK and India)
- Rice bran oil
- Safflower oil
- Olive oil
- Semi-refined sesame oil
- Semi-refined sunflower oil[29]
Less aggressive frying temperatures are frequently used.[30] A quality frying oil has a bland flavor, at least 200 °C (392 °F) smoke and 315 °C (599 °F) flash points, with maximums of 0.1% free fatty acids and 3% linolenic acid.[31] Those oils with higher linolenic fractions are avoided due to polymerization or gumming marked by increases in viscosity with age.[30] Olive oil resists thermal degradation and has been used as a frying oil for thousands of years.[30]
Storing and keeping oil
[edit]All oils degrade in response to heat, light, and oxygen.[32] To delay the onset of rancidity, a blanket of an inert gas, usually nitrogen, is applied to the vapor space in the storage container immediately after production – a process called tank blanketing.[citation needed][33]
In a cool, dry place, oils have greater stability, but may thicken, although they will soon return to liquid form if they are left at room temperature. To minimize the degrading effects of heat and light, oils should be removed from cold storage just long enough for use.[citation needed]
Refined oils high in monounsaturated fats, such as macadamia oil,[32] keep up to a year, while those high in polyunsaturated fats, such as soybean oil, keep about six months. Rancidity tests have shown that the shelf life of walnut oil is about 3 months, a period considerably shorter than the best before date shown on labels.[32]
By contrast, oils high in saturated fats, such as avocado oil, have relatively long shelf lives and can be safely stored at room temperature, as the low polyunsaturated fat content facilitates stability.[32]
Types and characteristics
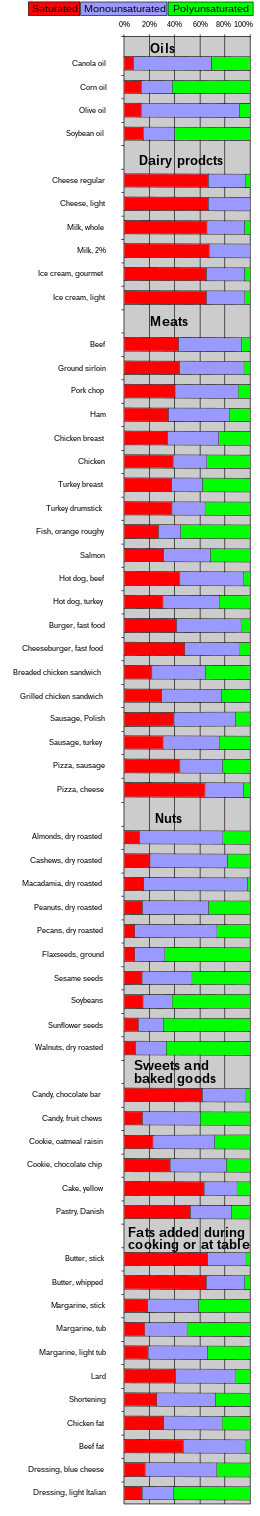
[edit]Cooking oils are composed of various fractions of fatty acids.[34] For the purpose of frying food, oils high in monounsaturated or saturated fats are generally popular, while oils high in polyunsaturated fats are less desirable.[24] High oleic acid oils include almond, macadamia, olive, pecan, pistachio, and high-oleic cultivars of safflower and sunflower.[35]
| Oils and fats | Saturated fatty acids | MUFA | PUFA | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4:0 | 6:0 | 8:0 | 10:0 | 12:0 | 14:0 | 16:0 | 18:0 | 20:0 | 22:0 | 24:0 | 16:1 | 18:1 | 20:1 | 22:1 | 18:2 | 18:3 | |
| Almond[36] | 6.5 | 1.7 | 0.6 | 69.4 | 17.4 | ||||||||||||
| Almond[37] | 1 | 5 | 77 | 17 | |||||||||||||
| Apricot kernel[36] | 5.8 | 0.5 | 1.5 | 58.5 | 29.3 | ||||||||||||
| Avocado[36] | 10.9 | 0.7 | 2.7 | 67.9 | 12.5 | 1 | |||||||||||
| Basil[38] | 8.5 | 11 | 24.5 | 54.5 | |||||||||||||
| Brazil nut[39] | 0.1 | 13.5 | 11.8 | 0.5 | 0.3 | 29.1 | 0.2 | 42.8 | 0.2 | ||||||||
| Butter[40] | 5.3 | 2.8 | 1.6 | 3.1 | 3.4 | 10.8 | 28.1 | 10.6 | 1.4 | 20.8 | 0.3 | 2 | |||||
| Butter, anhydrous[36] | 3.2 | 1.9 | 1.1 | 2.5 | 2.8 | 10 | 26.2 | 12.1 | 2.2 | 25 | 2.2 | 1.4 | |||||
| Canola[36] | 4.3 | 2.1 | 0.7 | 0.3 | 0.2 | 61.7 | 1.3 | 19 | 9.1 | ||||||||
| Canola[41] | 3.9 | 1.9 | 0.6 | 0.2 | 0.2 | 0.2 | 64.1 | 1 | 18.7 | 9.2 | |||||||
| Cashew[38] | 11.5 | 9 | 61 | 17 | |||||||||||||
| Cocoa butter[36] | 0.1 | 25.4 | 33.2 | 0.2 | 32.6 | 2.8 | 0.1 | ||||||||||
| Coconut[42] | 0.4 | 7.3 | 6.6 | 47.8 | 18.1 | 8.9 | 2.7 | 0.1 | 6.4 | 1.6 | |||||||
| Corn[36] | 10.6 | 1.8 | 0.4 | 0.1 | 27.3 | 0.1 | 53.5 | 1.2 | |||||||||
| Cottonseed[43] | 0.9 | 25.5 | 2.5 | 0.3 | 0.2 | 0.6 | 17.7 | 52.2 | 0.1 | ||||||||
| Grapeseed[36] | 0.1 | 6.7 | 2.7 | 0.3 | 15.8 | 69.6 | 0.1 | ||||||||||
| Hazelnut[39] | 0.1 | 5.8 | 2.7 | 0.2 | 0.3 | 79.3 | 0.2 | 10.4 | 0.5 | ||||||||
| Hemp[38] | 6.5 | 3 | 11.5 | 56.5 | 20 | ||||||||||||
| Lard[44] | 0.1 | 0.2 | 1.4 | 24.9 | 14.1 | 2.8 | 43.1 | 10.7 | 1 | ||||||||
| Macadamia nut[39] | 1 | 8.4 | 3.2 | 2.3 | 17.3 | 65.1 | 2.2 | 2.3 | 0.1 | ||||||||
| Olive[36] | 11.3 | 2 | 0.4 | 0.1 | 1.3 | 71.3 | 0.3 | 9.8 | 0.8 | ||||||||
| Olive, Virgin[45] | 13.8 | 2.8 | 0.1 | 1.9 | 69 | 12.2 | |||||||||||
| Palm kernel[46] | 0.3 | 3.6 | 3.3 | 48 | 16.7 | 8.5 | 2.1 | 14.9 | 2.5 | ||||||||
| Palm[47] | 0.3 | 1.1 | 43.5 | 4.3 | 0.2 | 0.2 | 39.8 | 10.2 | 0.3 | ||||||||
| Palm[36] | 0.1 | 1 | 43.5 | 4.3 | 0.3 | 36.6 | 0.1 | 9.1 | 0.2 | ||||||||
| Peanut[41] | 0.1 | 11.6 | 3.1 | 1.5 | 3 | 1 | 0.2 | 46.5 | 1.4 | 31.4 | |||||||
| Rapeseed[45] | 4.8 | 1.9 | 60.5 | 22.5 | 9.5 | ||||||||||||
| Rice bran[48] | 0.4 | 19.8 | 1.9 | 0.9 | 0.3 | 0.2 | 42.3 | 0.5 | 31.9 | 1.2 | |||||||
| Safflower, high oleic[36] | 0.1 | 4.9 | 1.9 | 0.4 | 0.3 | 0.1 | 74.8 | 0.3 | 12.7 | 0.1 | |||||||
| Safflower[49] | 7.3 | 2.5 | 13.6 | 75.7 | 0.5 | ||||||||||||
| Sesame[50] | 0.1 | 9.2 | 5.8 | 0.7 | 0.2 | 0.1 | 40.6 | 0.2 | 42.6 | 0.3 | |||||||
| Soybean[36] | 10.5 | 4.4 | 0.4 | 0.4 | 22.6 | 0.2 | 51 | 6.8 | |||||||||
| Soybean[41] | 0.1 | 11 | 4 | 0.3 | 0.1 | 0.1 | 23.4 | 53.2 | 7.8 | ||||||||
| Soybean, low linolenic[43] | 10.8 | 4.5 | 0.4 | 0.4 | 26.1 | 55.4 | 2 | ||||||||||
| Soybean, high oleic[note 1] | 7.3 | 3.4 | 0.4 | 0.4 | 85.1 | 1.3 | 2 | ||||||||||
| Sunflower[41] | 0.5 | 0.2 | 6.8 | 4.7 | 0.4 | 0.1 | 18.6 | 68.2 | 0.5 | ||||||||
| Sunflower, high linoleic[36] | 5.9 | 4.5 | 19.5 | 65.7 | |||||||||||||
| Sunflower, linoleic[36] | 5.4 | 3.5 | 0.2 | 45.3 | 39.8 | 0.2 | |||||||||||
| Sunflower, mid-oleic[36] | 0.1 | 4.2 | 3.6 | 0.3 | 0.8 | 0.1 | 57 | 0.2 | 28.9 | ||||||||
| Sunflower, high oleic[36] | 0.1 | 3.7 | 4.3 | 1 | 0.1 | 82.6 | 1 | 3.6 | 0.2 | ||||||||
| Sunflower, high oleic I[51] | 5 | 3 | 82 | 9 | |||||||||||||
| Sunflower, high oleic II[51] | 5 | 4 | 90 | 1 | |||||||||||||
| Tallow, beef[36] | 0.9 | 3.7 | 24.9 | 18.9 | 4.2 | 36 | 0.3 | 3.1 | 0.6 | ||||||||
| Tallow, mutton[36] | 3.8 | 21.5 | 19.5 | 2.3 | 37.6 | 5.5 | 2.3 | ||||||||||
| Walnut[39] | 0.1 | 6.7 | 2.3 | 0.1 | 0.2 | 21 | 0.2 | 57.5 | 11.6 | ||||||||
| [52] Parts per hundred | |||||||||||||||||
Smoke point
[edit]The smoke point is marked by "a continuous wisp of smoke".[53] It is the temperature at which an oil starts to burn, leading to a burnt flavor in the foods being prepared and degradation of nutrients and phytochemicals characteristic of the oil.[54]
Above the smoke point are flash and fire points.[53] The flash point is the temperature at which oil vapors will ignite but are not produced in sufficient quantities to stay lit. The flash point generally occurs at about 275–330 °C (527–626 °F).[55] The fire point is the temperature at which hot oil produces sufficient vapors they will catch on fire and burn.[55] As frying hours increase, all these temperature points decrease.[55] They depend more on an oil's acidity than fatty-acid profile.[56]
The smoke point of cooking oils varies generally in association with how oil is refined: a higher smoke point results from removal of impurities and free fatty acids.[54] Residual solvent remaining from the refining process may decrease the smoke point.[56] It has been reported to increase with the inclusion of antioxidants (BHA, BHT, and TBHQ). For these reasons, the published smoke points of oils may vary.[56]
| Fat | Quality | Smoke point[caution 1] | |
|---|---|---|---|
| Almond oil | 221 °C | 430 °F[57] | |
| Avocado oil | Refined | 271 °C | 520 °F[58][59] |
| Avocado oil | Unrefined | 250 °C | 482 °F[60] |
| Beef tallow | 250 °C | 480 °F | |
| Butter | 150 °C | 302 °F[61] | |
| Butter | Clarified | 250 °C | 482 °F[62] |
| Castor oil | Refined | 200 °C[63] | 392 °F |
| Coconut oil | Refined, dry | 204 °C | 400 °F[64] |
| Coconut oil | Unrefined, dry expeller pressed, virgin | 177 °C | 350 °F[64] |
| Corn oil | 230–238 °C[65] | 446–460 °F | |
| Corn oil | Unrefined | 178 °C[63] | 352 °F |
| Cottonseed oil | Refined, bleached, deodorized | 220–230 °C[66] | 428–446 °F |
| Flaxseed oil | Unrefined | 107 °C | 225 °F[59] |
| Grape seed oil | 216 °C | 421 °F | |
| Lard | 190 °C | 374 °F[61] | |
| Mustard oil | 250 °C | 480 °F[67] | |
| Olive oil | Refined | 199–243 °C | 390–470 °F[68] |
| Olive oil | Virgin | 210 °C | 410 °F |
| Olive oil | Extra virgin, low acidity, high quality | 207 °C | 405 °F[59][69] |
| Olive oil | Extra virgin | 190 °C | 374 °F[69] |
| Palm oil | Fractionated | 235 °C[70] | 455 °F |
| Peanut oil | Refined | 232 °C[59] | 450 °F |
| Peanut oil | 227–229 °C[59][71] | 441–445 °F | |
| Peanut oil | Unrefined | 160 °C[59] | 320 °F |
| Pecan oil | 243 °C[72] | 470 °F | |
| Rapeseed oil (Canola) | 220–230 °C[73] | 428–446 °F | |
| Rapeseed oil (Canola) | Expeller press | 190–232 °C | 375–450 °F[74] |
| Rapeseed oil (Canola) | Refined | 204 °C | 400 °F |
| Rapeseed oil (Canola) | Unrefined | 107 °C | 225 °F |
| Rice bran oil | Refined | 232 °C[48] | 450 °F |
| Safflower oil | Unrefined | 107 °C | 225 °F[59] |
| Safflower oil | Semirefined | 160 °C | 320 °F[59] |
| Safflower oil | Refined | 266 °C | 510 °F[59] |
| Sesame oil | Unrefined | 177 °C | 350 °F[59] |
| Sesame oil | Semirefined | 232 °C | 450 °F[59] |
| Soybean oil | 234 °C[75] | 453 °F | |
| Sunflower oil | Neutralized, dewaxed, bleached & deodorized | 252–254 °C[76] | 486–489 °F |
| Sunflower oil | Semirefined | 232 °C[59] | 450 °F |
| Sunflower oil | 227 °C[59] | 441 °F | |
| Sunflower oil | Unrefined, first cold-pressed, raw | 107 °C[77] | 225 °F |
| Sunflower oil, high oleic | Refined | 232 °C | 450 °F[59] |
| Sunflower oil, high oleic | Unrefined | 160 °C | 320 °F[59] |
| Vegetable oil blend | Refined | 220 °C[69] | 428 °F |
- ^ Specified smoke, fire, and flash points of any fat and oil can be misleading: they depend almost entirely upon the free fatty acid content, which increases during storage or use. The smoke point of fats and oils decreases when they are at least partially split into free fatty acids and glycerol; the glycerol portion decomposes to form acrolein, which is the major source of the smoke evolved from heated fats and oils. A partially hydrolyzed oil therefore smokes at a lower temperature than non-hydrolyzed oil. (Adapted from Gunstone, Frank D., ed. (17 March 2011). Vegetable Oils in Food Technology: Composition, Properties and Uses. Wiley, Inc. OCLC 1083187382.)
Oils are extracted from nuts, seeds, olives, grains or legumes by extraction using industrial chemicals or by mechanical processes. Expeller pressing is a chemical-free process that collects oils from a source using a mechanical press with minimal heat. Cold-pressed oils are extracted under a controlled temperature setting usually below 105 °C (221 °F) intended to preserve naturally occurring phytochemicals, such as polyphenols, tocotrienols, plant sterols and vitamin E which collectively affect color, flavor, aroma and nutrient value.[54][78]
| Type of oil or fat |
SFA | MUFA | PUFA | Omega- | Smoke point | Uses | |
|---|---|---|---|---|---|---|---|
| 3 | 6 | ||||||
| Almond | 8% | 66% | 26% | 0 | 17% | 221 °C (430 °F) | Baking, sauces, flavoring |
| Avocado oil | 12% | 74% | 14% | 0.95% | 12% | 271 °C (520 °F) | Frying, sautéing, dipping oil, salad oil |
| Butter | 66% | 30% | 4% | 0.3% | 2.7% | 150 °C (302 °F) | Cooking, baking, condiment, sauces, flavoring |
| Butter, clarified, Ghee | 65% | 32% | 3% | 0 | 0 | 190–250 °C (374–482 °F) | Deep frying, cooking, sautéing, condiment, flavoring |
| Canola oil | 6% | 62% | 32% | 9.1% | 18% | 225 °C (437 °F)[73] | Frying, baking, salad dressings |
| Coconut oil (virgin) | 92% | 6% | 2% | 0 | 1.8% | 177 °C (351 °F) | Cooking, tropical cuisine, beauty products |
| Corn oil | 13% | 25% | 62% | 1.1% | 53% | 235 °C (455 °F)[81] | Frying, baking, salad dressings, margarine, shortening |
| Cottonseed oil | 24% | 26% | 50% | 0.2% | 50% | 216 °C (421 °F) | Margarine, shortening, salad dressings, commercially fried products |
| Diacylglycerol (DAG) oil | 3.05% | 37.95% | 59% | 0 | - | 215 °C (419 °F) | Frying, baking, salad oil |
| Linseed oil[82] | 11% | 21% | 68% | 53% | 13% | 107 °C (225 °F) | Salad dressings, nutritional supplement |
| Grapeseed oil | 12% | 17% | 71% | 0.1% | 69% | 204 °C (399 °F) | Cooking, salad dressings, margarine |
| Hemp oil | 9% | 12% | 79% | 18% | 55% | 165 °C (329 °F) | Cooking, salad dressings |
| Lard | 41% | 47% | 2% | 1% | 10% | 183–205 °C (361–401 °F) | Baking, frying |
| Macadamia oil | 12.5% | 84% | 3.5% | 0 | 2.8% | 210 °C (410 °F) | Cooking, frying, deep frying, salads, dressings. A slightly nutty odour. |
| Margarine (hard) | 80% | 14% | 6% | 2% | 22% | 150 °C (302 °F) | Cooking, baking, condiment |
| Margarine (soft) | 20% | 47% | 33% | 2.4% | 23% | 150–160 °C (302–320 °F) | Cooking, baking, condiment |
| Mustard oil | 13% | 60% | 21% | 5.9% | 15% | 254 °C (489 °F) | Cooking, frying, deep frying, salads, dressings. Very clean flavoured & palatable. |
| Olive oil (extra virgin) | 14% | 73% | 11% | 0.7% | 9.8% | 190 °C (374 °F) | Cooking, salad oils, margarine |
| Olive oil (virgin) | 14% | 73% | 11% | 0.7% | 9.8% | 215 °C (419 °F) | Cooking, salad oils, margarine |
| Olive oil (refined) | 14% | 73% | 11% | 0 | 0 | 225 °C (437 °F) | Sautee, stir frying, deep frying, cooking, salad oils, margarine |
| Olive oil (extra light) | 14% | 73% | 11% | 0 | 0 | 242 °C (468 °F) | Sautee, stir frying, frying, deep frying, cooking, salad oils, margarine |
| Palm oil | 52% | 38% | 10% | 0.2% | 9.1% | 230 °C (446 °F) | Frying,[83] cooking, flavoring, vegetable oil, shortening |
| Peanut oil | 18% | 49% | 33% | 0 | 31% | 231 °C (448 °F) | Frying, cooking, salad oils, margarine, deep frying |
| Pumpkin seed oil | 8% | 36% | 57% | 0% | 64% | 121 °C (250 °F) | Salad oils |
| Rice bran oil | 20% | 47% | 33% | 1.6% | 33% | 213 °C (415 °F)[48] | Cooking, frying, deep frying, salads, dressings. Very clean flavoured & palatable. |
| Safflower oil (high oleic)[84][85] | 6% | 75% | 13% | 242 °C (468 °F)[81] | Frying, cooking | ||
| Safflower oil (linoleic)[86] | 6% | 14% | 75% | 242 °C (468 °F)[81] | Cooking, salad dressings, margarine | ||
| Sesame oil (unrefined) | 14% | 43% | 43% | 0.3 | 41% | 177 °C (351 °F) | Cooking |
| Sesame oil (semi-refined) | 14% | 43% | 43% | 0.3 | 41% | 232 °C (450 °F) | Cooking, deep frying |
| Soybean oil | 15% | 24% | 61% | 6.7% | 50% | 240 °C (464 °F)[81] | Cooking, salad dressings, vegetable oil, margarine, shortening |
| Sunflower oil (high oleic, refined)[87] | 9% | 82% | 9% | 0.2% | 3.6% | 244 °C (471 °F)[81] | Frying, cooking[88] |
| Sunflower oil (linoleic, refined)[87] | 11% | 20% | 69% | 0% | 56% | 240 °C (464 °F)[81] | Cooking, salad dressings, margarine, shortening |
| Sunflower oil (mid-oleic, refined, NuSun)[87] | 9% | 65% | 26% | 211 °C (412 °F)[81] | Commercial food manufacturing | ||
| Tea seed oil[89] | 22% | 60% | 18% | 0.7% | 22% | 252 °C (486 °F) | Cooking, salad dressings, stir frying, frying, margarine |
| Tallow[90] | 43% | 50% | 4% | 1% | 3% | 249 °C (480 °F) | Cooking, shortening, pemmican, deep frying |
| Walnut oil (semi-refined) | 9% | 23% | 63% | 10% | 53% | 204 °C (399 °F)[91] | Salad dressings, added to cold dishes to enhance flavor |
| [92] | |||||||
Extraction and refinement
[edit]This section needs additional citations for verification. (July 2021) |

Cooking oil extraction and refinement are separate processes. Extraction first removes the oil, typically from a seed, nut or fruit. Refinement then alters the appearance, texture, taste, smell, or stability of the oil to meet buyer expectations.
Extraction
[edit]There are three broad types of oil extraction:
- Chemical solvent extraction, most commonly using hexane.
- Pressing, using an expeller press or cold press (pressing at low temperatures to prevent oil heating).
- Decanter centrifuge.
In large-scale industrial oil extraction you will often see some combination of pressing, chemical extraction and/or centrifuging in order to extract the maximum amount of oil possible.[104]
Refinement
[edit]Cooking oil can either be unrefined, or refined using one or more of the following refinement processes (in any combination):[105]
- Distilling, which heats the oil to evaporate off chemical solvents from the extraction process.
- Degumming, by passing hot water through the oil to precipitate out gums and proteins that are soluble in water but not in oil, then discarding the water along with the impurities.
- Neutralization,[106] or deacidification, which treats the oil with sodium hydroxide or sodium carbonate to pull out free fatty acids, phospholipids, pigments, and waxes.
- Bleaching, which removes "off-colored" components by treatment with fuller's earth, activated carbon, or activated clays, followed by heating, filtering, then drying to recoup the oil.
- Dewaxing, or winterizing, improves clarity of oils intended for refrigeration by dropping them to low temperatures and removing any solids that form.
- Deodorizing, by treating with high-heat pressurized steam to evaporate less stable compounds that might cause "unusual" odors or tastes.[107]
- Preservative addition, including antioxidants such as BHA, BHT, and tocopherol to help preserve oils that have been made less stable due to high-temperature processing.
Filtering, a non-chemical process which screens out larger particles, could be considered a step in refinement, although it does not alter the state of the oil.
Most large-scale commercial cooking oil refinement will involve all of these steps in order to achieve a product that's uniform in taste, smell and appearance, and has a longer shelf life.[104] Cooking oil intended for the health food market will often be unrefined, which can result in a less stable product but minimizes exposure to high temperatures and chemical processing.
Waste cooking oil
[edit]
Proper disposal of used cooking oil is an important waste-management concern. Oil can congeal in pipes, causing sanitary sewer overflow.[108] Because of this, cooking oil should never be dumped in the kitchen sink or in the toilet bowl. The proper way to dispose of oil is to put it in a sealed non-recyclable container and discard it with regular garbage.[109] Placing the container of oil in the refrigerator to harden also makes disposal easier and less messy.
Recycling
[edit]Cooking oil can be recycled. It can be used in animal feed, soap, make-up, clothes, rubber, detergents, directly as fuel, and to produce biodiesel.[110][111][112]
In the recycling industry, used cooking oil recovered from restaurants and food-processing industries (typically from deep fryers or griddles) is called yellow grease, recycled vegetable oil (RVO), used vegetable oil (UVO), or waste vegetable oil (WVO).[113]
Grease traps or interceptors collect fats and oils from kitchen sinks and floor drains. The result is called brown grease, and unlike yellow grease its severe contaminants make it much harder to recycle.
Adulteration
[edit]Gutter oil and trench oil are terms used in China to describe recycled oil processed to resemble virgin oil, but containing toxic contaminants and sold illegally for cooking; its origin is frequently brown grease from garbage.[114]
In Kenya, thieves sell stolen electric transformers to operators of roadside food stalls for reuse of the oil in deep frying, suitable for prolonged use longer than regular cooking oil, but a threat to consumer health due to the presence of PCBs and polycyclic aromatic hydrocarbons.[115]
References
[edit]- ^ "Dietary fats explained". Retrieved August 5, 2018.
- ^ Yanai H, Katsuyama H, Hamasaki H, et al. (2015). "Effects of Dietary Fat Intake on HDL Metabolism". J Clin Med Res. 7 (3): 145–9. doi:10.14740/jocmr2030w. PMC 4285059. PMID 25584098.
- ^ Clarke, R; Frost, C; Collins, R; et al. (1997). "Dietary lipids and blood cholesterol: quantitative meta-analysis of metabolic ward studies". BMJ. 314 (7074): 112–7. doi:10.1136/bmj.314.7074.112. PMC 2125600. PMID 9006469.
- ^ Mensink, RP; Zock, PL; Kester, AD; Katan, MB (2003). "Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials". Am J Clin Nutr. 77 (5): 1146–55. doi:10.1093/ajcn/77.5.1146. PMID 12716665.
- ^ Jakobsen, M. U; O'Reilly, E. J; Heitmann, B. L; et al. (2009). "Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies". American Journal of Clinical Nutrition. 89 (5): 1425–32. doi:10.3945/ajcn.2008.27124. PMC 2676998. PMID 19211817.
- ^ a b Katan, Martijn B.; Mozaffarian, Dariush; Micha, Renata; Wallace, Sarah (2010). Katan, Martijn B. (ed.). "Effects on Coronary Heart Disease of Increasing Polyunsaturated Fat in Place of Saturated Fat: A Systematic Review and Meta-Analysis of Randomized Controlled Trials". PLOS Medicine. 7 (3): e1000252. doi:10.1371/journal.pmed.1000252. PMC 2843598. PMID 20351774.
- ^ "Dietary fats: Know which types to choose". Mayo Clinic Staff. 2015.
- ^ "Choose foods low in saturated fat". National Heart, Lung, and Blood Institute (NHLBI), NIH Publication No. 97-4064. 1997. Archived from the original on 2009-12-13. Retrieved 2009-09-28.
- ^ Kabagambe, EK; Baylin, A; Ascherio, A & Campos, H (November 2005). "The Type of Oil Used for Cooking Is Associated with the Risk of Nonfatal Acute Myocardial Infarction in Costa Rica". Journal of Nutrition. 135 (11) (135 ed.): 2674–2679. doi:10.1093/jn/135.11.2674. PMID 16251629.
- ^ Urry. Campbell Biology. Pearson.
- ^ Sands, David C.; Morris, Cindy E.; Dratz, Edward A.; Pilgeram, Alice (2009). "Elevating optimal human nutrition to a central goal of plant breeding and production of plant-based foods". Plant Sci. 117 (5): 377–389. doi:10.1016/j.plantsci.2009.07.011. PMC 2866137. PMID 20467463.
- ^ Food and nutrition board, institute of medicine of the national academies (2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academies Press. p. 423. ISBN 978-0-309-08537-3.
- ^ Food and nutrition board, institute of medicine of the national academies (2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academies Press. p. 504. ISBN 978-0-309-08537-3.[permanent dead link]
- ^ "Trans fat: Avoid this cholesterol double whammy". Mayo Foundation for Medical Education and Research (MFMER). Retrieved 2007-12-10.
- ^ Mozaffarian, Dariush; Katan, Martijn B.; Ascherio, Alberto; et al. (2006). "Trans Fatty Acids and Cardiovascular Disease". New England Journal of Medicine. 354 (15): 1601–113. doi:10.1056/NEJMra054035. PMID 16611951.
- ^ Willett, WC; Stampfer, MJ; Manson, JE; et al. (1993). "Intake of trans fatty acids and risk of coronary heart disease among women". Lancet. 341 (8845): 581–5. doi:10.1016/0140-6736(93)90350-P. PMID 8094827. S2CID 2616254.
- ^ Hu, Frank B.; Stampfer, Meir J.; Manson, Joann E.; et al. (1997). "Dietary Fat Intake and the Risk of Coronary Heart Disease in Women". New England Journal of Medicine. 337 (21): 1491–9. doi:10.1056/NEJM199711203372102. PMID 9366580.
- ^ Hayakawa, Kyoko; Linko, Yu-Yen; Linko, Pekka (2000). "The role of trans fatty acids in human nutrition". Starch - Stärke. 52 (6–7): 229–35. doi:10.1002/1521-379X(200007)52:6/7<229::AID-STAR229>3.0.CO;2-G.
- ^ The Nurses' Health Study (NHS)
- ^ "Final Determination Regarding Partially Hydrogenated Oils (Removing Trans Fat)". Food and Drug Administration. Retrieved March 29, 2019.
- ^ Doureradjou, P.; Koner, Bidhan Chandra (2008). "Effect of Different Cooking Vessels on Heat Induced Lipid Peroxidation of Different Edible Oils". Journal of Food Biochemistry. 32 (6): 740–751. doi:10.1111/j.1745-4514.2008.00195.x. ISSN 1745-4514.
- ^ a b Crosby (2018) Do Cooking Oils Present a Health Risk?
- ^ Orna Izakson. "Oil right: choose wisely for heart-healthy cooking - Eating Right". E: The Environmental Magazine.
- ^ a b Bouchon, Pedro (2009). "Chapter 5 - Understanding Oil Absorption During Deep-Fat Frying". Advances in Food and Nutrition Research. 57: 209–34. doi:10.1016/S1043-4526(09)57005-2. ISBN 9780123744401. ISSN 1043-4526. PMID 19595388.
- ^ De Marco, Elena; Savarese, Maria; Parisini, Cristina; Battimo, Ilaria; Falco, Salvatore; Sacchi, Raffaele (2007). "How to make sunflower oil?". Huatai Oil Machinery. 109 (3): 237–246. doi:10.1002/ejlt.200600192. Archived from the original on August 19, 2024.
- ^ Che Man, YB; Liu, J.L.; Jamilah, B.; Rahman, R. Abdul (1999). "Quality changes of RBD palm olein, soybean oil and their blends during deep-fat frying". Journal of Food Lipids. 6 (3): 181–193. doi:10.1111/j.1745-4522.1999.tb00142.x.
- ^ Matthäus, Bertrand (2007). "Use of palm oil for frying in comparison with other high-stability oils". European Journal of Lipid Science and Technology. 109 (4): 400–409. doi:10.1002/ejlt.200600294.
- ^ Sundram, K; Sambanthamurthi, R; Tan, YA (2003). "Palm fruit chemistry and nutrition" (PDF). Asia Pacific Journal of Clinical Nutrition. 12 (3): 355–62. PMID 14506001.
- ^ "Smoke Points of Various Fats - Kitchen Notes - Cooking For Engineers". cookingforengineers.com. 2012. Retrieved July 3, 2012.
- ^ a b c Boskou, Dimitrios (2010). "21 Frying Fats". Chemical and functional properties of food lipids. p. 429.
- ^ Rossell, J.B. (1998). "Industrial frying process". Grasas y Aceites. 49 (3–4): 282–295. doi:10.3989/gya.1998.v49.i3-4.729.
- ^ a b c d Kochhar, S. Parkash; Henry, C. Jeya K. (2009-01-01). "Oxidative stability and shelf-life evaluation of selected culinary oils". International Journal of Food Sciences and Nutrition. 60 (Suppl 7): 289–296. doi:10.1080/09637480903103774. ISSN 1465-3478. PMID 19634067. S2CID 44352150.
- ^ Mishra, Sundeep; Manchanda, S.C. (Feb 2012). "Cooking oils for heart health" (PDF). J Prev Cardiol. 1 (3): 123–131. Archived from the original (PDF) on 2022-08-14. Retrieved 2022-06-21 – via Google Scholar.
- ^ Kuo, T.M.; Gardner, H. (2002). Lipid Biotechnology. Taylor & Francis. p. 1. ISBN 9780824744182. LCCN 2001058440.
- ^ The Lipid Handbook (2007), p. 4.
- ^ a b c d e f g h i j k l m n o p q r "National Nutrient Database for Standard Reference Release 28" (PDF). USDA.
- ^ R.S. Guad; S.J. Surana; G.S. Talele; S.G. Talele; S.B. Gokhale (2006). Natural Excipients. Pragati Books Pvt. Ltd. ISBN 978-81-85790-60-2.
- ^ a b c The Lipid Handbook (2007), pp. 71–73.
- ^ a b c d Vegetable Oils in Food Technology (2011), p. 329.
- ^ The Lipid Handbook (2007), pp. 93.
- ^ a b c d Vegetable Oils in Food Technology (2011), p. 61.
- ^ Vegetable Oils in Food Technology (2011), p. 172.
- ^ a b c Warner, K.; Gupta, M. (2005). "Potato chip quality and frying oil stability of high oleic acid soybean oil". Journal of Food Science. 70 (6).
- ^ The Lipid Handbook (2007), pp. 98.
- ^ a b Vegetable Oils in Food Technology (2011), p. 141.
- ^ Vegetable Oils in Food Technology (2011), p. 180.
- ^ Sundram, K; Sambanthamurthi, R; Tan, YA (2003). "Palm fruit chemistry and nutrition" (PDF). Asia Pacific Journal of Clinical Nutrition. 12 (3): 355–62. PMID 14506001.
- ^ a b c Vegetable Oils in Food Technology (2011), p. 303.
- ^ Takeuchi, H.; Matsuo, T.; Tokuyama, K.; Shimomura, Y.; Suzuki, M. "Diet-induced thermogenesis is lower in rats fed a lard diet than in those fed a high oleic acid safflower oil diet, a safflower oil diet or a linseed oil diet". The Journal of Nutrition. 125 (4): 920.
- ^ Vegetable Oils in Food Technology (2011), p. 293.
- ^ a b Vegetable Oils in Food Technology (2011), p. 148.
- ^ "Fats and fatty acids".
- ^ a b Vegetable Oils in Food Technology (2011), p. 122.
- ^ a b c Beck, Leslie (28 September 2015). "'Smoke point' matters when cooking with oil". Toronto, Canada: The Globe and Mail. Retrieved 11 April 2017.
- ^ a b c Vegetable Oils in Food Technology (2011), p. 90.
- ^ a b c Vegetable Oils in Food Technology (2011), p. 149.
- ^ Marcus, Jacqueline B. (2013). Culinary Nutrition: The Science and Practice of Healthy Cooking. Academic Press. p. 61. ISBN 978-012-391882-6.
Table 2-3 Smoke Points of Common Fats and Oils
. - ^ "Smoking Points of Fats and Oils". What’s Cooking America.
- ^ a b c d e f g h i j k l m n o "Smoke Point of Oils". Baseline of Health. Jonbarron.org. 2012-04-17. Retrieved 2019-12-26.
- ^ Marie Wong; Cecilia Requejo-Jackman; Allan Woolf (April 2010). "What is unrefined, extra virgin cold-pressed avocado oil?". Aocs.org. Retrieved 26 December 2019.
- ^ a b The Culinary Institute of America (2011). The Professional Chef (9th ed.). Hoboken, New Jersey: John Wiley & Sons. ISBN 978-0-470-42135-2. OCLC 707248142.
- ^ "Smoke Point of different Cooking Oils". Charts Bin. 2011.
- ^ a b Detwiler, S. B.; Markley, K. S. (1940). "Smoke, flash, and fire points of soybean and other vegetable oils". Oil & Soap. 17 (2): 39–40. doi:10.1007/BF02543003.
- ^ a b "Introducing Nutiva Organic Refined Coconut Oil". Nutiva. Archived from the original on 2015-02-14.
- ^ Vegetable Oils in Food Technology (2011), p. 284.
- ^ Vegetable Oils in Food Technology (2011), p. 214.
- ^ "Mustard Seed Oil". Clovegarden.
- ^ "Olive Oil Smoke Point". Retrieved 2016-08-25.
- ^ a b c Gray, S (June 2015). "Cooking with extra virgin olive oil" (PDF). ACNEM Journal. 34 (2): 8–12.
- ^ (in Italian) Scheda tecnica dell'olio di palma bifrazionato PO 64.
- ^ Vegetable Oils in Food Technology (2011), p. 234.
- ^ Ranalli N, Andres SC, Califano AN (Jul 2017). "Dulce de leche‐like product enriched with emulsified pecan oil: Assessment of physicochemical characteristics, quality attributes, and shelf‐life". European Journal of Lipid Science and Technology. doi:10.1002/ejlt.201600377. Retrieved 15 January 2021.
- ^ a b Vegetable Oils in Food Technology (2011), p. 121.
- ^ "What is the "truth" about canola oil?". Spectrum Organics, Canola Oil Manufacturer. Archived from the original on April 13, 2014.
- ^ Vegetable Oils in Food Technology (2011), p. 92.
- ^ Vegetable Oils in Food Technology (2011), p. 153.
- ^ "Organic unrefined sunflower oil". Retrieved 18 December 2016.
- ^ Ramadan, Mohamed (2020). Cold Pressed Oils: Green Technology, Bioactive Compounds, and Applications. Academic Press. p. 311. ISBN 9780128181898.
- ^ F. D. Gunstone; D. Rousseau (2004). Rapeseed and canola oil: production, processing, properties and uses. Oxford: Blackwell Publishing Ltd. p. 91. ISBN 0-8493-2364-9. Retrieved 2011-01-17.
- ^ Brown, Amy L. (2010). Understanding Food: Principles and Preparation. Belmont, CA: Wadsworth Publishing. p. 468. ISBN 0-538-73498-1. Retrieved 2011-01-16.
The smoke point of an oil depends primarily on its free fatty acid content (FFA) and molecular weight. Through repeated use, as in a deep fryer, the oil accumulates food residues or by-products of the cooking process, that lower its smoke point further. The values shown in the table must therefore be taken as approximate, and are not suitable for accurate or scientific use
- ^ a b c d e f g Kodali, D.R. (ed.). Trans Fats Replacement Solutions. AOCS Press. p. 143. ISBN 978-0-9830791-5-6.
- ^ A. G. Vereshagin and G. V. Novitskaya (1965) The triglyceride composition of linseed oil. Journal of the American Oil Chemists' Society 42, 970-974. [1]
- ^ Rossell, J.B. (1998). "Industrial frying process" (PDF). Grasas y Aceites. 49 (3–4): 282–295.
- ^ National nutrient database for standard reference release 28. "Basic Report: 04511, Oil, safflower, salad or cooking, high oleic (primary safflower oil of commerce)". USDA.[dead link]
- ^ "Smoke point of oils". Jonbarron.org. Retrieved April 10, 2017.
- ^ National nutrient database for standard reference release 28. "Basic Report: 04510, Oil, safflower, salad or cooking, linoleic, (over 70%)". USDA.[dead link]
- ^ a b c [2]
- ^ Abidi, S. L.; Warner, K. (2001). "Molecular-Weight Distributions of Degradation Products in Selected Frying Oils". JAOCS. 78 (7).
- ^ "Triglyceride composition of tea seed oil". doi:10.1002/jsfa.2740271206.
- ^ National Research Council, 1976, Fat Content and Composition of Animal Products, Printing and Publishing Office, National Academy of Science, Washington, D.C., ISBN 0-309-02440-4; p. 203, online edition
- ^ "Cooking Oil Smoke Points". Retrieved January 3, 2011.
- ^ "List of Abbreviations". The Journal of Nutrition. Retrieved April 18, 2017.
- ^ "Thrive Culinary Algae Oil". Retrieved 7 January 2019.
- ^ a b c d e f Anderson D. "Fatty acid composition of fats and oils" (PDF). Colorado Springs: University of Colorado, Department of Chemistry. Retrieved April 8, 2017.
- ^ "NDL/FNIC Food Composition Database Home Page". United States Department of Agriculture, Agricultural Research Service. Retrieved May 21, 2013.
- ^ "Basic Report: 04042, Oil, peanut, salad or cooking". USDA. Archived from the original on March 9, 2016. Retrieved 16 January 2015.
- ^ "Oil, vegetable safflower, oleic". nutritiondata.com. Condé Nast. Retrieved 10 April 2017.
- ^ "Oil, vegetable safflower, linoleic". nutritiondata.com. Condé Nast. Retrieved 10 April 2017.
- ^ "Oil, vegetable, sunflower". nutritiondata.com. Condé Nast. Retrieved 27 September 2010.
- ^ USDA Basic Report Cream, fluid, heavy whipping
- ^ "Nutrition And Health". The Goose Fat Information Service.
- ^ "Egg, yolk, raw, fresh". nutritiondata.com. Condé Nast. Retrieved 24 August 2009.
- ^ "09038, Avocados, raw, California". National Nutrient Database for Standard Reference, Release 26. United States Department of Agriculture, Agricultural Research Service. Archived from the original on January 10, 2014. Retrieved 14 August 2014.
- ^ a b "How cooking oil is made". Retrieved May 18, 2012.
- ^ Martin, Geoffrey (1920). Animal and Vegetable Oils, Fats, & Waxes: Their Manufacture, Refining, and Analysis, including the Manufacture of Candles, Margarine, and Butter. Crosby Lockwood and Son. pp. 79-80.
- ^ Vegetable Oils in Food Technology (2011), p. 236.
- ^ US 110626, Bradley, Henry W., "Improvement in compounds for culinary use", published 1871-01-03
- ^ "Tips to avoid water waste and to require the preservation of hydro-resources". Natureba - Educação Ambiental. Retrieved 2007-09-05.
- ^ "Grease Disposal Tips to Help the City's Environment". NYC Department of Environmental Protection. Retrieved 2007-08-05.
- ^ "Production of biodiesel based on waste oils and/or waste fats from biogenic origin for use as fuel" (PDF). CDM - Executive Board. Archived from the original (PDF) on 2007-09-27. Retrieved 2007-09-05.
- ^ Murphy, Denis J. Plant lipids: biology, utilisation, and manipulation. Wiley-Blackwell, 2005, p. 117.
- ^ Radich, Anthony Biodiesel Performance, Costs, and Use Archived 2022-06-26 at the Wayback Machine
- ^ Brown Grease Feedstocks for Biodiesel Archived 2012-06-17 at the Wayback Machine. K. Shaine Tyson, National Renewable Energy Laboratory. Retrieved January 31, 2009
- ^ Austin Ramzy (13 September 2011). "China Cracks Down on "Gutter Oil," a Substance Even Worse Than its Name". Time Inc. Retrieved 13 March 2016.
- ^ Iraki XN (12 December 2014). "Thieves fry Kenya's power grid for fast food". Al Jazeera Media Network. Retrieved 13 March 2016.
- The Lipid Handbook (2007). Frank D. Gunstone; John L. Harwood; Albert J. Dijkstra (eds.). The Lipid Handbook with CD-ROM (Third ed.). CRC Press. ISBN 978-0-8493-9688-5.
- Vegetable Oils in Food Technology (2011). Frank D. Gunstone (ed.). Vegetable Oils in Food Technology -- Composition, Properties and Uses (Second ed.). Blackwell Publishing Ltd. ISBN 978-1-4443-3268-1.
Further reading
[edit]- Warner, K. (1999). "Impact of High-Temperature Food Processing on Fats and Oils". Impact of Processing on Food Safety. Advances in Experimental Medicine and Biology. Vol. 459. pp. 67–77. doi:10.1007/978-1-4615-4853-9_5. ISBN 978-1-4613-7201-1. PMID 10335369.
- Fox, R. (2001). Frying oils. In Kaarin Goodburn (Ed.) EU Food Law. Woodhead. pp. 195–224. ISBN 978-1-85573-557-6.