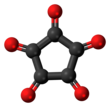
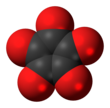
Cyclopentanepentone
Appearance
(Redirected from C5O5)

| |||
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
Cyclopentane-1,2,3,4,5-pentone[1] | |||
| Other names
Leuconic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5O5 | |||
| Molar mass | 140.050 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Cyclopentanepentone, also known as leuconic acid, is a hypothetical organic compound with formula C5O5, the fivefold ketone of cyclopentane. It would be an oxide of carbon (an oxocarbon), indeed a pentamer of carbon monoxide.
As of 2000, the compound had yet to be synthesized in bulk, but there have been reports of trace synthesis.[2][3][4]
Related compounds
[edit]Cyclopentanepentone can be viewed as the neutral counterpart of the croconate anion C
5O2−
5.
The compound referred to in the literature and trade as "cyclopentanepentone pentahydrate" (C5O5·5H2O) is probably decahydroxycyclopentane (C5(OH)10).[3][5]
References
[edit]- ^ "CID 12305030 - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ^ Rubin, M. B.; Gleiter, R. (2000). "The Chemistry of Vicinal Polycarbonyl Compounds". Chemical Reviews. 100 (3): 1121–64. doi:10.1021/cr960079j. PMID 11749259.
- ^ a b Seitz, G.; Imming, P. (1992). "Oxocarbons and pseudooxocarbons". Chemical Reviews. 92 (6): 1227–1260. doi:10.1021/cr00014a004.
- ^ Schröder, D.; Schwarz, H.; Dua, S.; Blanksby, S. J.; Bowie, J. H. (1999). "Mass spectrometric studies of the oxocarbons CnOn (n = 3–6)". International Journal of Mass Spectrometry. 188 (1–2): 17–25. Bibcode:1999IJMSp.188...17S. doi:10.1016/S1387-3806(98)14208-2.
- ^ Person, W. B.; Williams, D. G. (1957). "Infrared Spectra and the Structure of Leuconic Acid and Triquinoyl". Journal of Physical Chemistry. 61 (7): 1017–1018. doi:10.1021/j150553a047.